Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser.
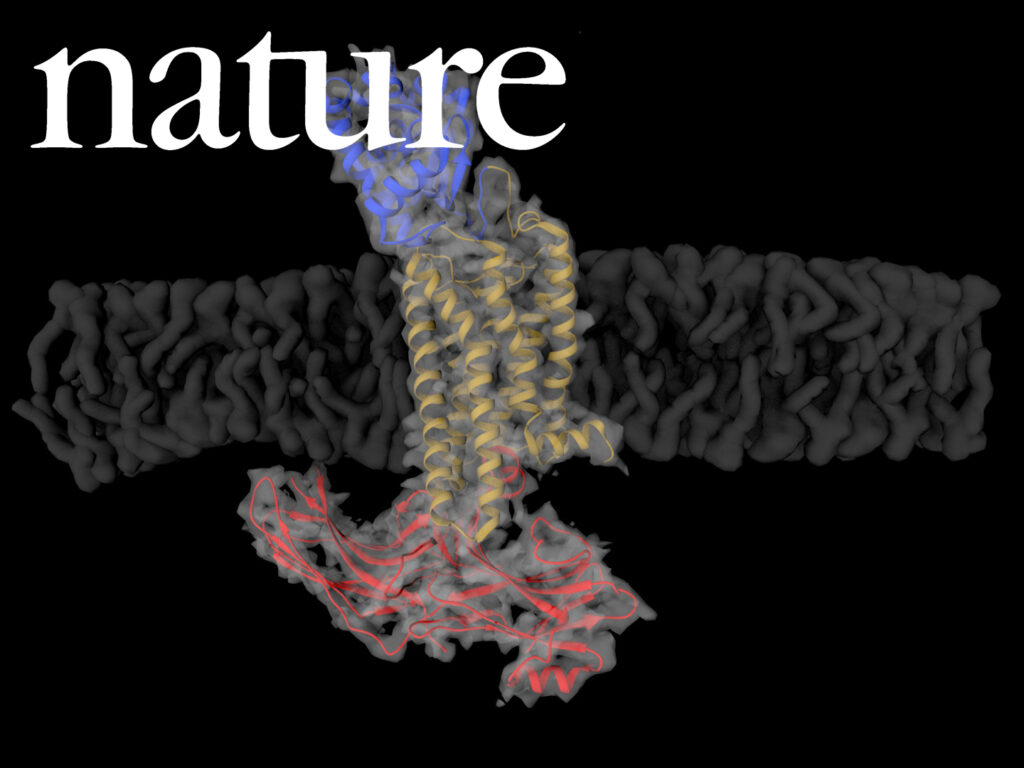
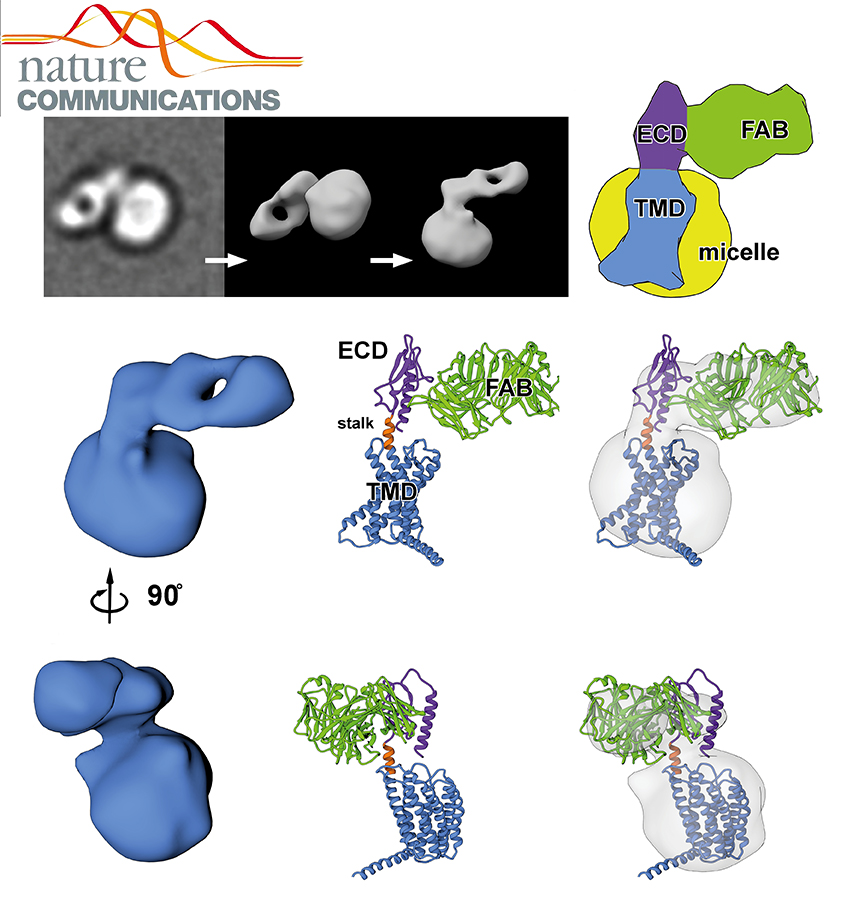
G-protein-coupled receptors (GPCRs) signal primarily through G proteins or arrestins. Arrestin binding to GPCRs blocks G protein interaction and redirects signalling to numerous G-protein-independent pathways. Here we report the crystal structure of a constitutively active form of human rhodopsin bound to a pre-activated form of the mouse visual arrestin, determined by serial femtosecond X-ray laser crystallography.
Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Read More »