Cotranslational assembly of membrane protein/nanoparticles in cell-free systems
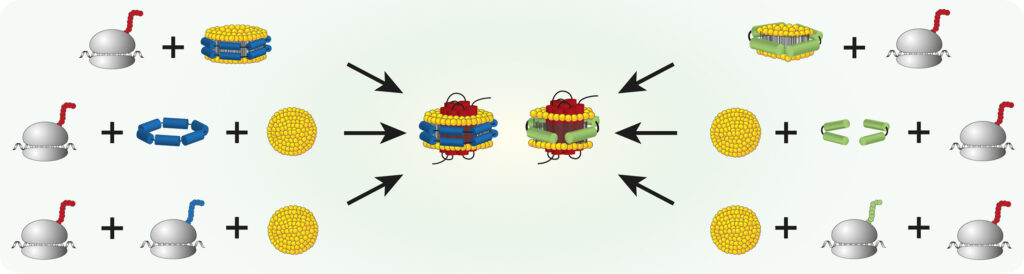
Roman Levin, Zoe Köck, Janosch Martin, René Zangl, Theresa Gewering, Leah Schüler, Arne Moeller, Volker Dötsch, Nina Morgner, Frank Bernhard Nanoparticles composed of amphiphilic scaffold proteins and small lipid bilayers are valuable tools for reconstitution and subsequent functional and structural characterization of membrane proteins. In combination with cell-free protein production systems, nanoparticles can be used […]
Cotranslational assembly of membrane protein/nanoparticles in cell-free systems Read More »