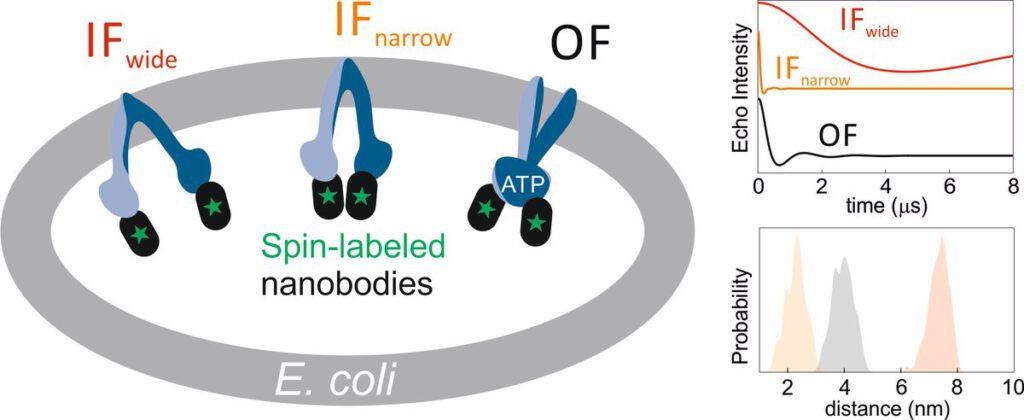
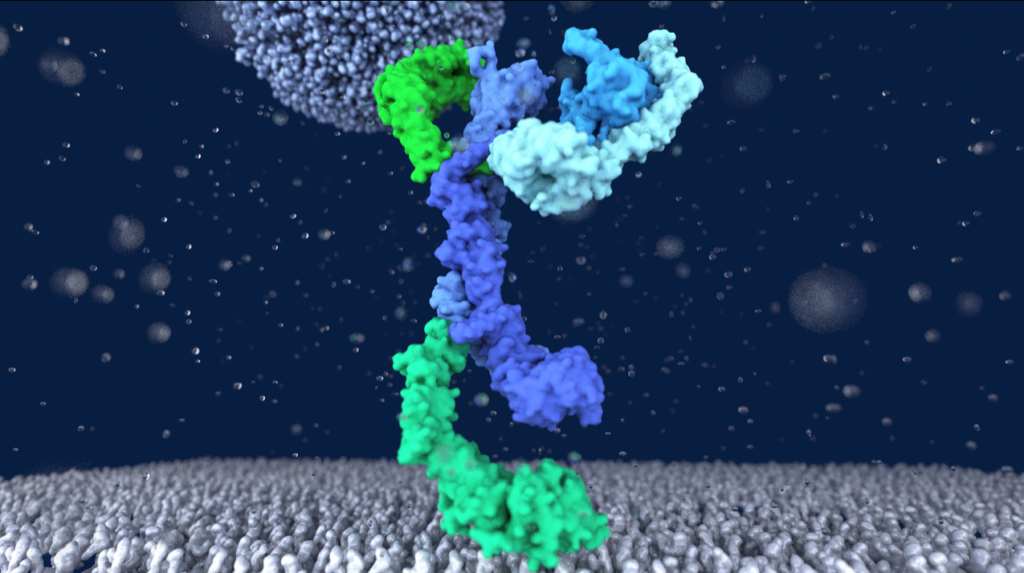
The ABC transporter MsbA adopts the wide inward-open conformation in E. coli cells
Laura Galazzo, Gianmarco Meier, Dovile Januliene, Kristian Parey, Dario De Vecchis, Bianca Striednig, Hubert Hilbi, Lars V. Schäfer, Ilya Kuprov, Arne Moeller, Enrica Bordignon, Markus A. Seeger Membrane proteins are currently investigated after detergent extraction from native cellular membranes and reconstitution into artificial liposomes or nanodiscs, thereby removing them from their physiological environment. However, to […]
The ABC transporter MsbA adopts the wide inward-open conformation in E. coli cells Read More »