Jan-Hannes Schäfer Lena Clausmeyer, Carolin Körner, Bianca M. Esch , Verena N. Wolf , Stefan Walter, Dovile Januliene, Arne Moeller, Florian Fröhlich
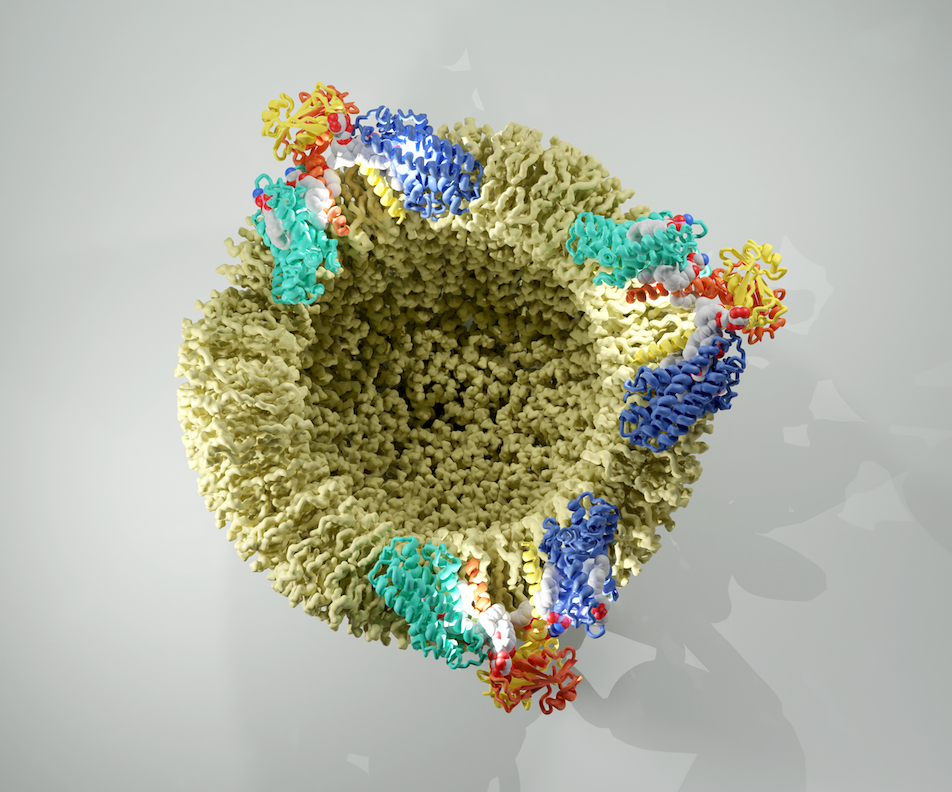
Ceramides play a pivotal role as essential lipids, serving as foundational components for complex sphingolipids and potent signaling molecules. Ceramides are the products of the N-acylation of a sphingoid base and a CoA-activated fatty acid. This reaction is catalyzed by the enzymes of the evolutionarily conserved ceramide synthase (CerS) family. Yet, the precise structural details and catalytic mechanisms of CerSs have remained elusive. Here, we employed cryo-EM single particle analysis to unravel the structure of the yeast ceramide synthase complex in both an active and a fumonisin B1 inhibited state. Our findings shed light on the complex’s architecture, revealing a dimer of Lip1 subunits bound to the two catalytic subunits, Lag1 and Lac1. Each catalytic subunit forms a hydrophobic crevice that is accessible from both the cytosolic site as well as from the intermembrane space of the endoplasmic reticulum (ER). Within this cavity, we identify amino acids forming the active center and a sphingoid base, one of the substrates of the complex. Together, this suggests a pre-loaded state of the CerS. Additionally, the fumonisin B1 bound structure reveals the inhibitory mechanism by blocking the cytosolic acyl-CoA binding site.