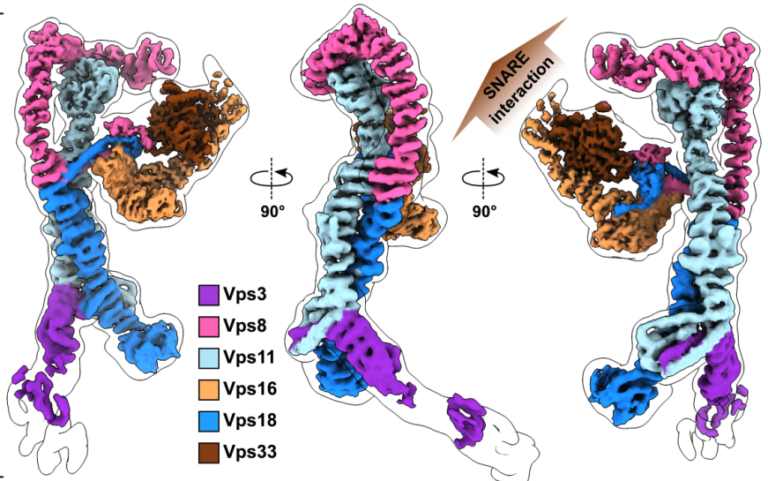
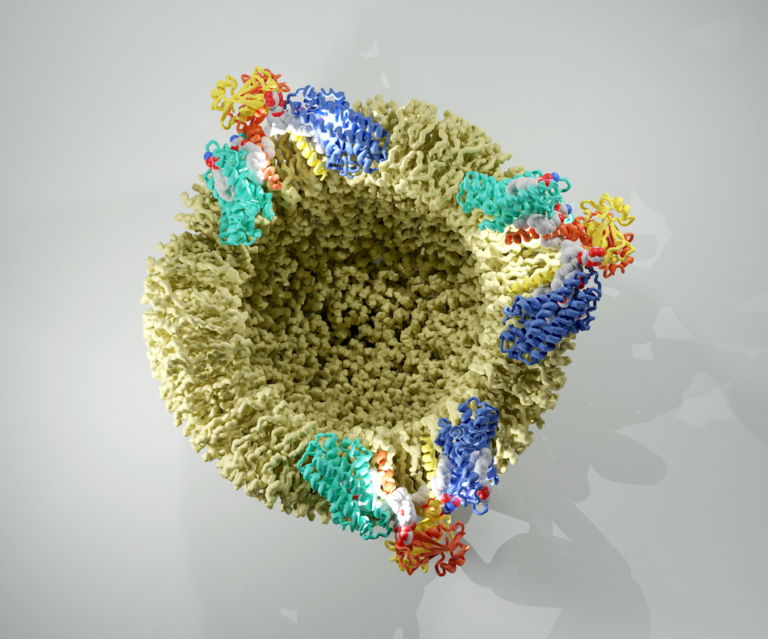
Structure of the endosomal CORVET tethering complex
Dmitry Shvarev, Caroline König, Nicole Susan, Lars Langemeyer, Stefan Walter, Angela Perz, Florian Fröhlich, Christian Ungermann, Arne Moeller Cells depend on their endolysosomal system for
The structure of the Orm2-containing serine palmitoyltransferase complex reveals distinct inhibitory potentials of yeast Orm proteins.
Carolin Körner, Jan-Hannes Schäfer, Bianca M. Escha , Kristian Parey, Stefan Walter, David Teis, Dovile Januliene, Oliver Schmidte, Arne Moellerb, Florian Fröhlich The levels of
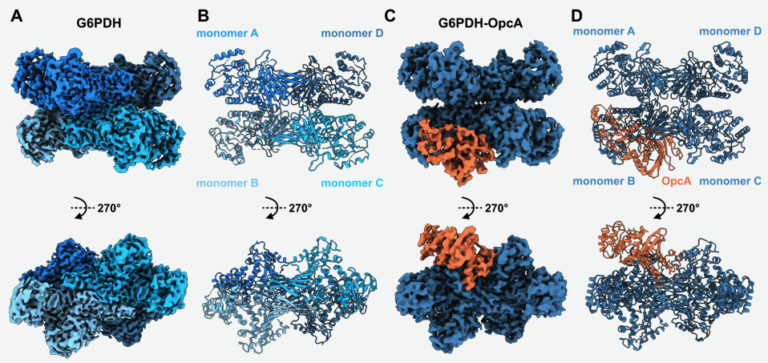
Structural basis of the allosteric regulation of cyanobacterial glucose-6-phosphate dehydrogenase by the redox sensor OpcA
Sofia Doello, Dmitry Shvarev, Marius Theune , Jakob Sauerwein, Alexander Klon, Erva Keskin, Marko Boehm, Kirstin Gutekunst, and Karl Forchhammera The oxidative pentose phosphate (OPP)
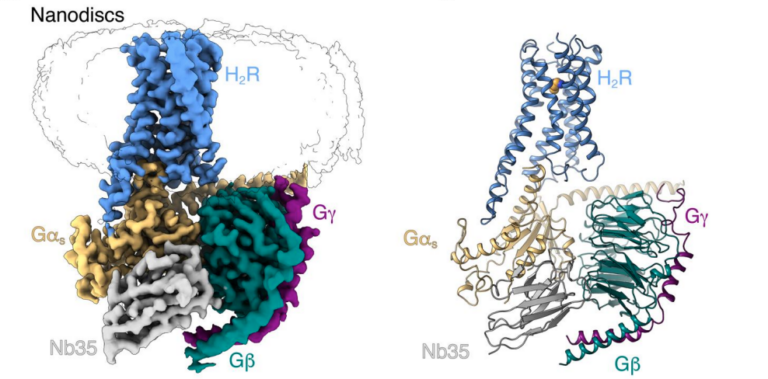
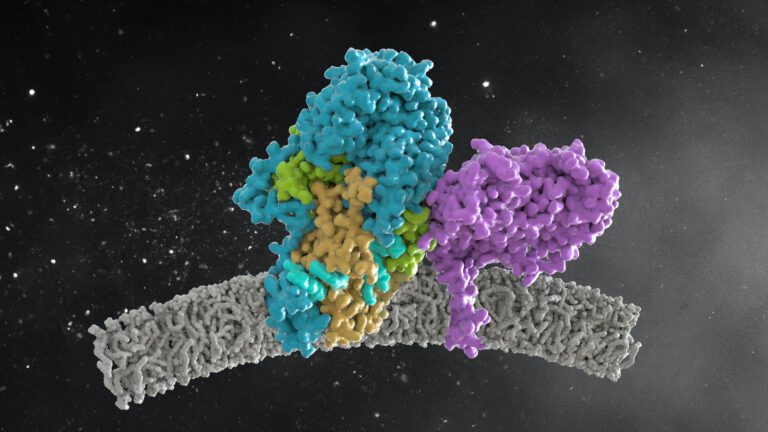
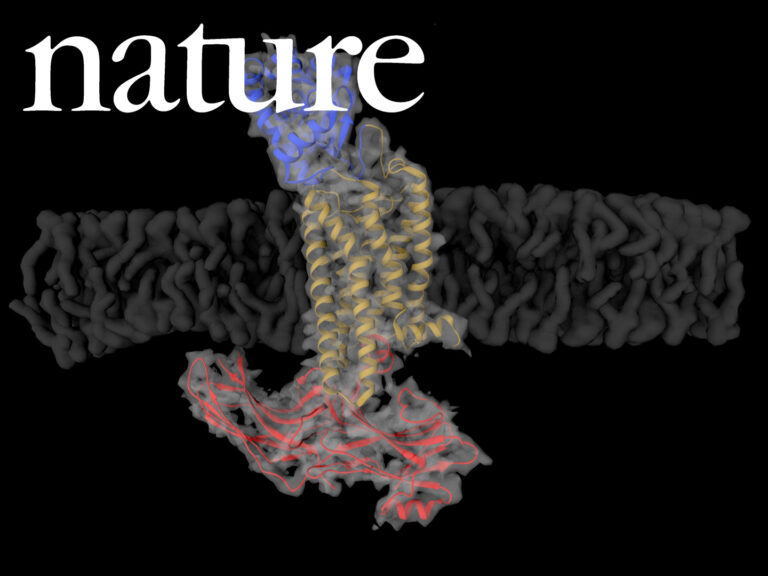
Cryo-EM structure of cell-free synthesized human histamine H2 receptor coupled to heterotrimeric Gs protein in lipid nanodisc environment
Zoe Köck, Kilian Schnelle, Margherita Persechino, Simon Umbach, Hannes Schihada, Dovile Januliene, Kristian Parey, Steffen Pockes, Peter Kolb, Volker Dötsch, ArneMöller, Daniel Hilger, Frank Bernhard
Structure of the yeast ceramide synthase
Jan-Hannes Schäfer Lena Clausmeyer, Carolin Körner, Bianca M. Esch , Verena N. Wolf , Stefan Walter, Dovile Januliene, Arne Moeller, Florian Fröhlich Ceramides play a
Structure of the ceramide-bound SPOTS complex
Jan-Hannes Schäfer, Carolin Körner, Bianca M. Esch, Sergej Limar, KristianParey, Stefan Walter, Dovile Januliene#, Arne Moeller#, Florian Fröhlich# Sphingolipids are structural membrane components that also
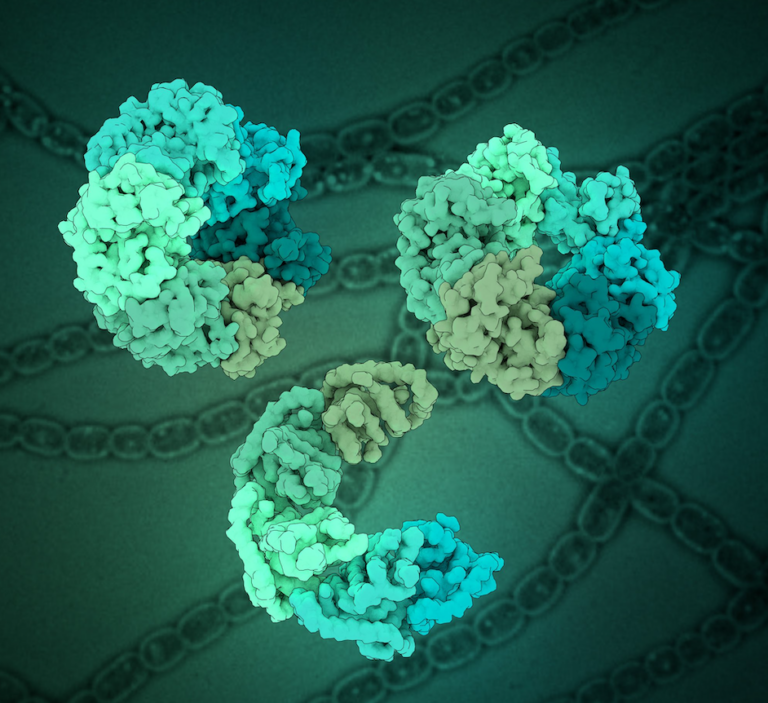
Conformational variability of cyanobacterial ChlI, the AAA+ motor of magnesium chelatase involved in chlorophyll biosynthesis
Dmitry Shvarev, Alischa Ira Scholz, Arne Moeller Magnesium chelatase is a conserved enzyme complex responsible for the first committed step of chlorophyll biosynthesis in photosynthetic

Yck3 casein kinase-mediated phosphorylation determines Ivy1 localization and function at endosomes and vacuole
Sophie Grziwa, Jan-Hannes Schäfer, Raffaele Nicastro, Annabel Arens, Claudio De Virgilio, Florian Fröhlich, Arne Moeller, Jieqiong Gao, Lars Langemeyer, Christian Ungermann The casein kinase Yck3
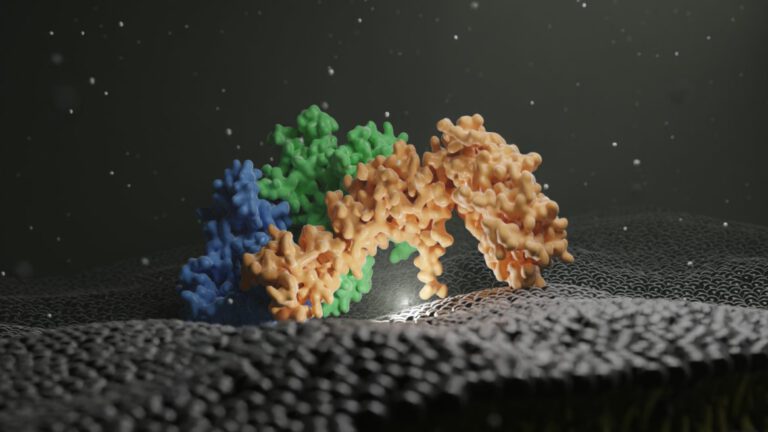
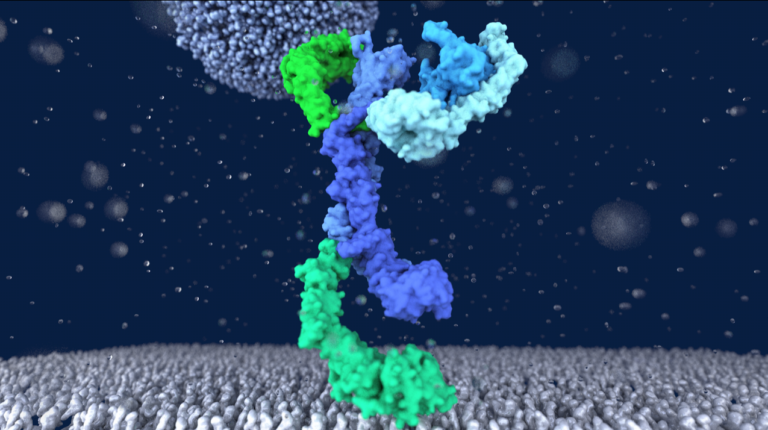
Structure of the metazoan Rab7 GEF complex Mon1–Ccz1–Bulli
Eric Herrmann, Jan-Hannes Schäfer, Stephan Wilmes, Christian Ungermann, Arne Moeller, and Daniel Kümmel The endosomal system of eukaryotic cells represents a central sorting and recycling
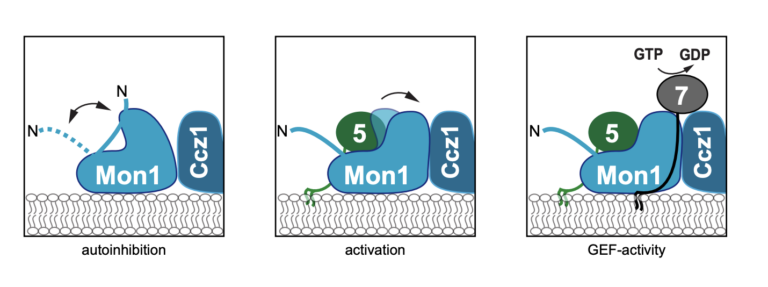
Regulatory sites in the Mon1-Ccz1 complex control Rab5 to Rab7 transition and endosome maturation
Ann-Christin Borchers, Maren Janz, Jan-Hannes Schäfer, Arne Moeller, Daniel Kümmel, Achim Paululat, Christian Ungermann, Lars Langemeyer Maturation from early to late endosomes depends on the
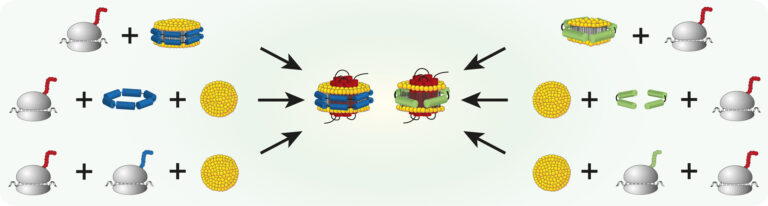
Cotranslational assembly of membrane protein/nanoparticles in cell-free systems
Roman Levin, Zoe Köck, Janosch Martin, René Zangl, Theresa Gewering, Leah Schüler, Arne Moeller, Volker Dötsch, Nina Morgner, Frank Bernhard Nanoparticles composed of amphiphilic scaffold
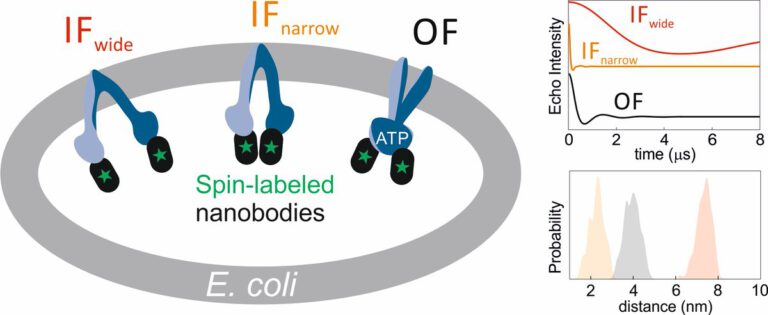
The ABC transporter MsbA adopts the wide inward-open conformation in E. coli cells
Laura Galazzo, Gianmarco Meier, Dovile Januliene, Kristian Parey, Dario De Vecchis, Bianca Striednig, Hubert Hilbi, Lars V. Schäfer, Ilya Kuprov, Arne Moeller, Enrica Bordignon, Markus
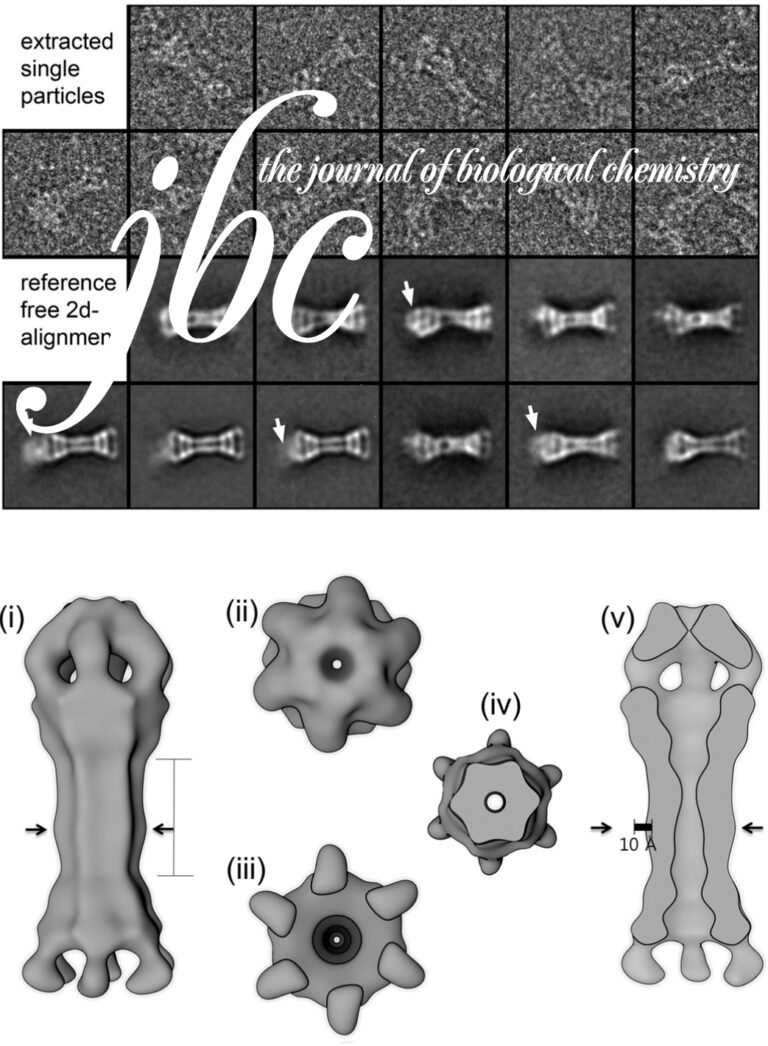
Structure of the HOPS tethering complex, a lysosomal membrane fusion machinery
Dmitry Shvarev, Jannis Schoppe, Caroline König, Angela Perz, Nadia Füllbrunn, Stephan Kiontke, Lars Langemeyer, Dovile Januliene, Kilian Schnelle, Daniel Kümmel, Florian Fröhlich, Arne Moeller, Christian
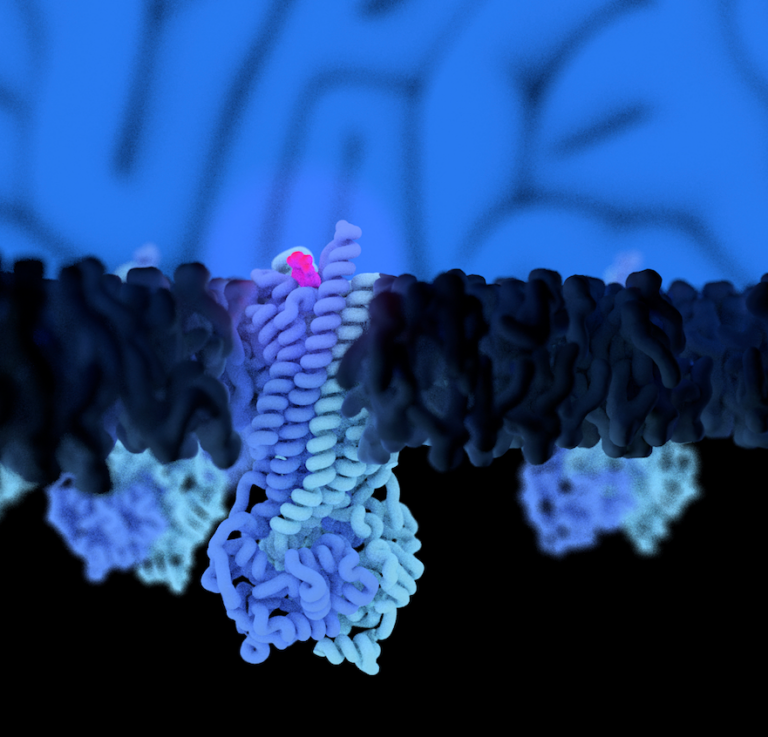
Mechanism of multimodal substrate translocation in P-glycoprotein
Theresa Gewering, Deepali Waghray, Kristian Parey, Joel Zapata, Pengyi Zhao, Hao Chen, Dovile Januliene, Ina L. Urbatsch, Arne Moeller, Qinghai Zhang P-glycoprotein (Pgp) is a
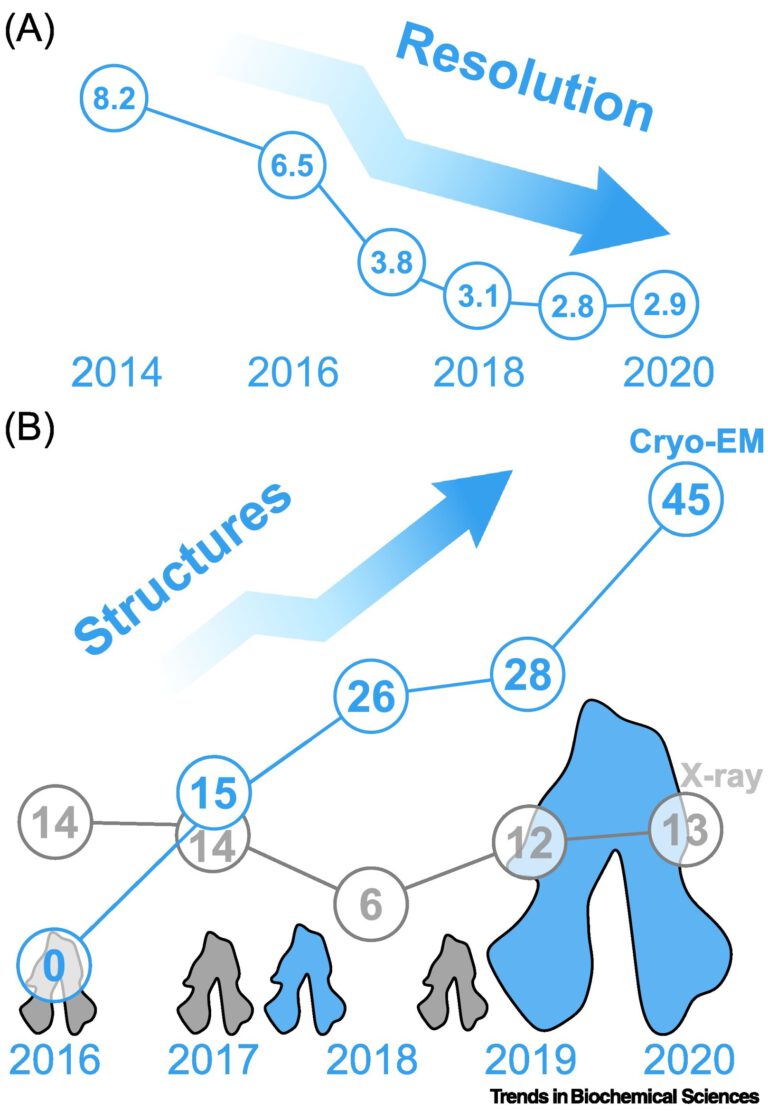
Frozen motion: how cryo-EM changes the way we look at ABC transporters
Dmitry Shvarev, Dovile Januliene, Arne Moeller ATP-binding cassette (ABC) transporters are widely present molecular machines that transfer substrates across the cell membrane. ABC transporters are
Single-Particle Cryo-EM of Membrane Proteins
Dovile Januliene and Arne Möller In the recent years, the protein databank has been fueled by the exponential growth of high-resolution electron cryo-microscopy (cryo-EM) structures.

Cryo‐EM of ABC transporters: an ice‐cold solution to everything?
Dovile Januliene & Arne Moeller High-resolution cryo-EM has revolutionized how we look at ABC transporters and membrane proteins in general. An ever-increasing number of software

Neue Möglichkeiten für die Strukturbestimmungvon Membranproteinen
Theresa Gewering, Arne Möller Membrane proteins establish the connection between the outside andthe inside of a cell. Even though 30 % of proteins in a

Structure of Merkel cell polyomavirus capsid and interaction with its 2 glycosaminoglycan attachment receptor
Merkel cell polyomavirus (MCPyV) is a human double-stranded DNA tumor virus. MCPyV cell entry is unique among the polyomavirus family as it requires the engagement of two types of glycans, sialylated oligosaccharides and sulfated glycosaminoglycans (GAGs). Here, we present crystallographic and cryo-electron microscopic structures of the icosahedral MCPyV capsid and analysis of its glycan interactions via NMR spectroscopy.
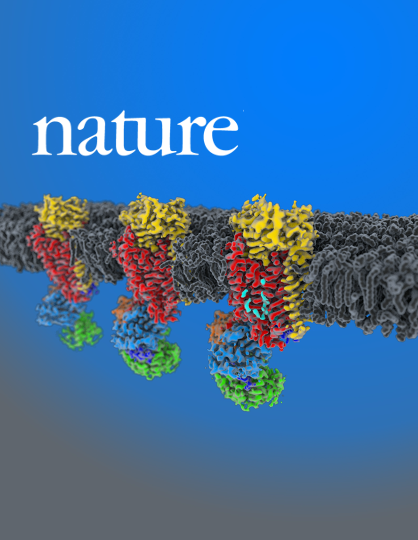
Structure and autoregulation of a P4-ATPase lipid flippase
Type 4 P-type ATPases (P4-ATPases) are lipid flippases that drive the active transport of phospholipids from exoplasmic or luminal leaflets to cytosolic leaflets of eukaryotic membranes. The molecular architecture of P4-ATPases and the mechanism through which they recognize and transport lipids have remained unknown.

Conformation space of a heterodimeric ABC exporter under turnover conditions.
Our findings reveal that phosphate release, not ATP hydrolysis, triggers the return of the exporter to the IF conformation. By mapping the conformational landscape during active turnover, aided by mutational and chemical modulation of kinetic rates to trap the key intermediates, we resolved fundamental steps of the substrate translocation cycle of asymmetric ABC transporters.
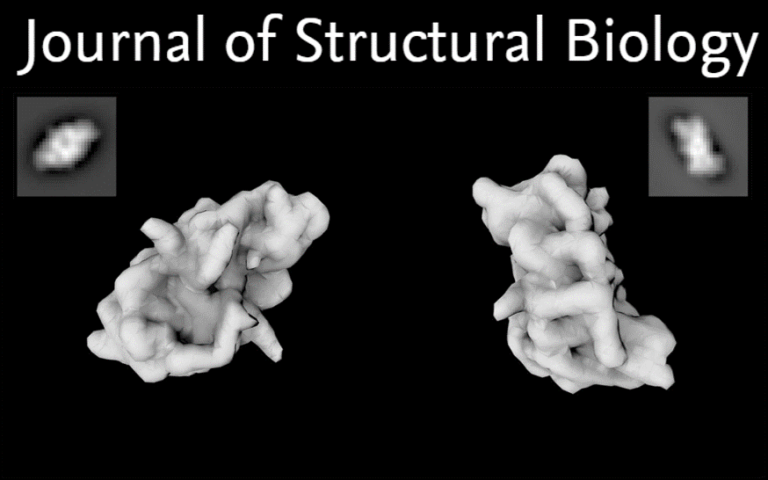
Know your detergents: A case study on detergent background in negative stain electron microscopy
Electron cryo-microscopy (cryo-EM) of purified macromolecular complexes is now providing 3D-structures at near-atomic resolution (Kühlbrandt, 2014). Cryo-EM can tolerate heterogeneous specimens, however, high-resolution efforts demand highly optimized samples.
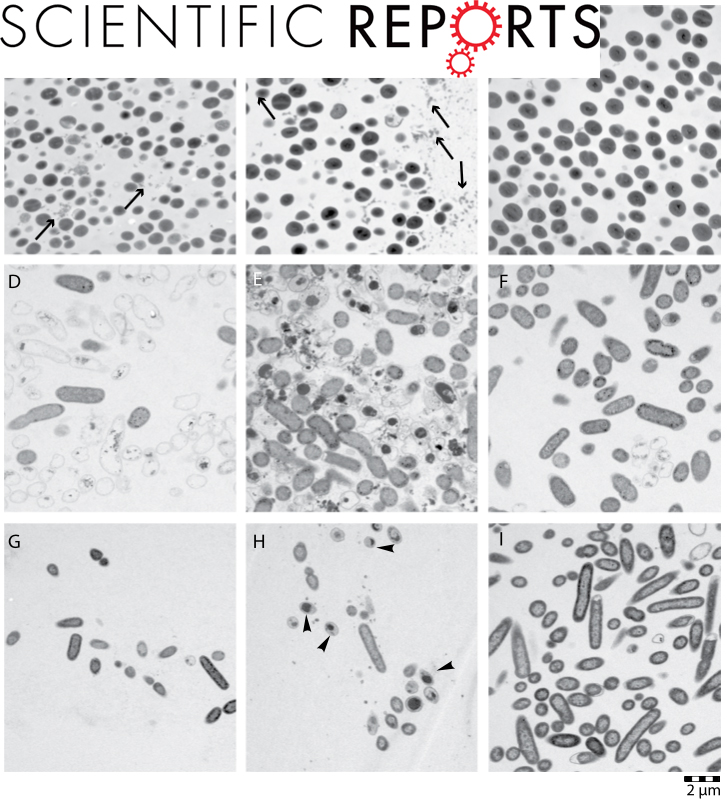
The Immunomodulatory Drug Glatiramer Acetate is Also an Effective Antimicrobial Agent that Kills Gram-negative Bacteria
Classic drug development strategies have failed to meet the urgent clinical needs in treating infections with Gram-negative bacteria. Repurposing drugs can lead to timely availability of new antibiotics, accelerated by existing safety profiles. Glatiramer acetate (GA) is a widely used and safe formulation for treatment of multiple sclerosis.
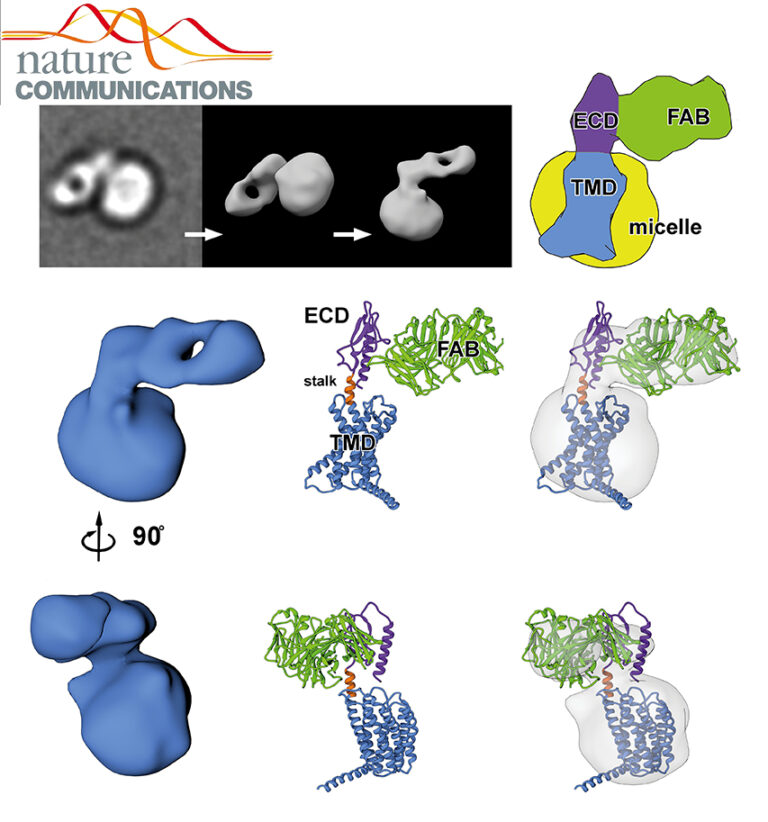
Structure of the human MHC-I peptide-loading complex
The peptide-loading complex (PLC) is a transient, multisubunit membrane complex in the endoplasmic reticulum that is essential for establishing a hierarchical immune response. The PLC coordinates peptide translocation into the endoplasmic reticulum with loading and editing of major histocompatibility complex class I (MHC-I) molecules.
Cryo-EM structure of the replisome reveals multiple interactions coordinating DNA synthesis
The antiparallel nature of the two strands in duplex DNA poses a topological problem for their simultaneous synthesis. The “trombone” model of the replication fork postulates that the lagging-strand forms a loop such that the leading- and lagging-strand replication proteins contact one another.

Acidic Environment Induces Dimerization and Ligand Binding Site Collapse in the Vps10p Domain of Article Acidic Environment Induces Dimerization and Ligand Binding Site Collapse in the Vps10p Domain of Sortilin
Sortilin is a neuronal receptor involved in transmembrane signaling, endocytosis, and intracellular sorting of proteins. It cycles through a number of cellular compartments where it encounters various acidic conditions. The crystal structure of the sortilin ectodomain has previously been determined at neutral pH. Here, we present the 3.5-Å resolution crystal structure of sortilin at pH 5.5, which represents an environment similar to that of late endosomes, where ligands are released.
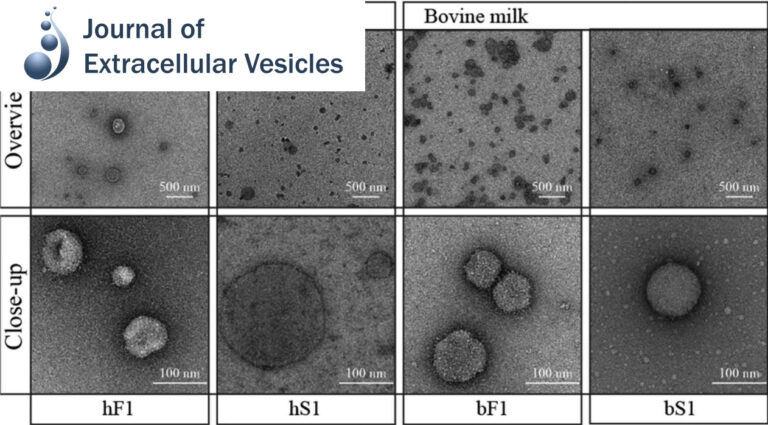
Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography.
Studies have suggested that nanoscale extracellular vesicles (EV) in human and bovine milk carry immune modulatory properties which could provide beneficial health effects to infants. In order to assess the possible health effects of milk EV, it is essential to use isolates of high purity from other more abundant milk structures with well-documented bioactive properties.
Hidden Twins: SorCS neuroreceptors form stable dimers. J Mol Biol
SorCS1, SorCS2 and SorCS3 belong to the Vps10p-domain family of multiligand receptors. Genetic and functional studies have linked SorCS receptors to psychiatric disorders, Alzheimer’s disease and type 2 diabetes, demonstrating critical roles in neuronal functionality and metabolic control.

Division of labor in transhydrogenase by alternating proton translocation and hydride transfer.
NADPH/NADP+ (the reduced form of NADP+/nicotinamide adenine dinucleotide phosphate) homeostasis is critical for countering oxidative stress in cells. Nicotinamide nucleotide transhydrogenase (TH), a membrane enzyme present in both bacteria and mitochondria, couples the proton motive force to the generation of NADPH. We present the 2.8 Å crystal structure of the transmembrane proton channel domain of TH from Thermus thermophilus and the 6.9 Å crystal structure of the entire enzyme (holo-TH).
Distinct conformational spectrum of homologous multidrug ABC transporters.
ATP-binding cassette (ABC) exporters are ubiquitously found in all kingdoms of life and their members play significant roles in mediating drug pharmacokinetics and multidrug resistance in the clinic. Here, we used single particle electron microscopy (EM) to delineate the entire conformational spectrum of two homologous ABC exporters (bacterial MsbA and mammalian P-glycoprotein) and the influence of nucleotide and substrate binding.
TRIP13 is a protein-remodeling AAA+ ATPase that catalyzes MAD2 conformation switching.
The AAA+ family ATPase TRIP13 is a key regulator of meiotic recombination and the spindle assembly checkpoint, acting on signaling proteins of the conserved HORMA domain family. Here we present the structure of the Caenorhabditis elegans TRIP13 ortholog PCH-2, revealing a new family of AAA+ ATPase protein remodelers.
Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser.
G-protein-coupled receptors (GPCRs) signal primarily through G proteins or arrestins. Arrestin binding to GPCRs blocks G protein interaction and redirects signalling to numerous G-protein-independent pathways. Here we report the crystal structure of a constitutively active form of human rhodopsin bound to a pre-activated form of the mouse visual arrestin, determined by serial femtosecond X-ray laser crystallography.
Conformational states of the full-length glucagon receptor.
Class B G protein-coupled receptors are composed of an extracellular domain (ECD) and a seven-transmembrane (7TM) domain, and their signalling is regulated by peptide hormones. Using a hybrid structural biology approach together with the ECD and 7TM domain crystal structures of the glucagon receptor (GCGR), we examine the relationship between full-length receptor conformation and peptide ligand binding.
Allosteric communication in the dynein motor domain.
Dyneins power microtubule motility using ring-shaped, AAA-containing motor domains. Here, we report X-ray and electron microscopy (EM) structures of yeast dynein bound to different ATP analogs, which collectively provide insight into the roles of dynein’s two major ATPase sites, AAA1 and AAA3, in the conformational change mechanism.
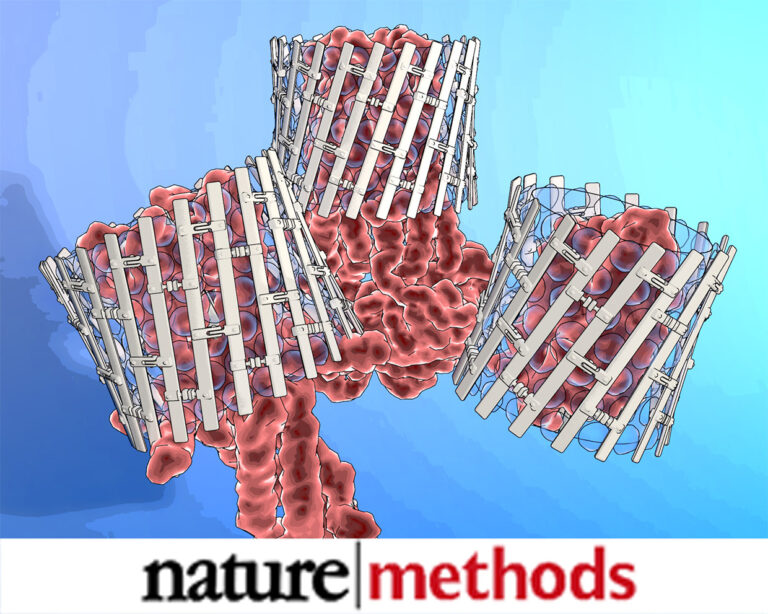
Engineered nanostructured β-sheet peptides protect membrane proteins.
We designed β-strand peptides that stabilize integral membrane proteins (IMPs). β-strand peptides self-assemble in solution as filaments and become restructured upon association with IMPs; resulting IMP–β-strand peptide complexes resisted aggregation when diluted in detergent-free buffer and were visible as stable, single particles with low detergent background in electron micrographs.
Nucleotide-dependent conformational changes in the N-Ethylmaleimide Sensitive Factor (NSF) and their potential role in SNARE complex disassembly.
Homohexameric, N-Ethylmaleimide Sensitive Factor (NSF) disassembles Soluble NSF Attachment Protein Receptor (SNARE) complexes after membrane fusion, an essential step in vesicular trafficking. NSF contains three domains (NSF-N, NSF-D1, and NSF-D2), each contributing to activity. We combined electron microscopic (EM) analysis, analytical ultracentrifugation (AU) and functional mutagenesis to visualize NSF’s ATPase cycle.
Assembly and channel opening of outer membrane protein in tripartite drug efflux pumps of Gram-negative bacteria.
In this work, we describe the crystal structure of the membrane fusion protein MexA from the Pseudomonas aeruginosa MexAB-OprM pump in the hexameric ring arrangement. Electron microscopy study on the chimeric complex of MexA and the outer membrane protein OprM reveals that MexA makes a tip-to-tip interaction with OprM, which suggests a docking model for MexA and OprM.

Movies of ice-embedded particles enhance resolution in electron cryo-microscopy.
Low-dose images obtained by electron cryo-microscopy (cryo-EM) are often affected by blurring caused by sample motion during electron beam exposure, degrading signal especially at high resolution. We show here that we can align frames of movies, recorded with a direct electron detector during beam exposure of rotavirus double-layered particles, thereby greatly reducing image blurring caused by beam-induced motion and sample stage instabilities.

Organization of the influenza virus replication machinery.
Influenza virus ribonucleoprotein complexes (RNPs) are central to the viral life cycle and in adaptation to new host species. RNPs are composed of the viral genome, viral polymerase, and many copies of the viral nucleoprotein. In vitro cell expression of all RNP protein components with four of the eight influenza virus gene segments enabled structural determination of native influenza virus RNPs by means of cryogenic electron microscopy (cryo-EM).
Functional implications of an intermeshing cogwheel-like interaction between TolC and MacA in the action of macrolide-specific efflux pump MacAB-TolC.
The inner membrane transporter MacB requires the outer membrane factor TolC and the periplasmic adaptor protein MacA to form a functional tripartite complex. In this study, we used a chimeric protein containing the tip region of the TolC α-barrel to investigate the role of the TolC α-barrel tip region with regard to its interaction with MacA.
Recombinant functional multidomain hemoglobin from the gastropod Biomphalaria glabrata.
The extracellular hemoglobin multimer of the planorbid snail Biomphalaria glabrata , intermediate host of the human parasite Schistosoma mansoni , is presumed to be a 1.44 MDa complex of six 240 kDa polypeptide subunits, arranged as three disulfide‐bridged dimers. The complete amino acid sequence of two subunit types (BgHb1 and BgHb2), and the partial sequence of a third type (BgHb3) are known.
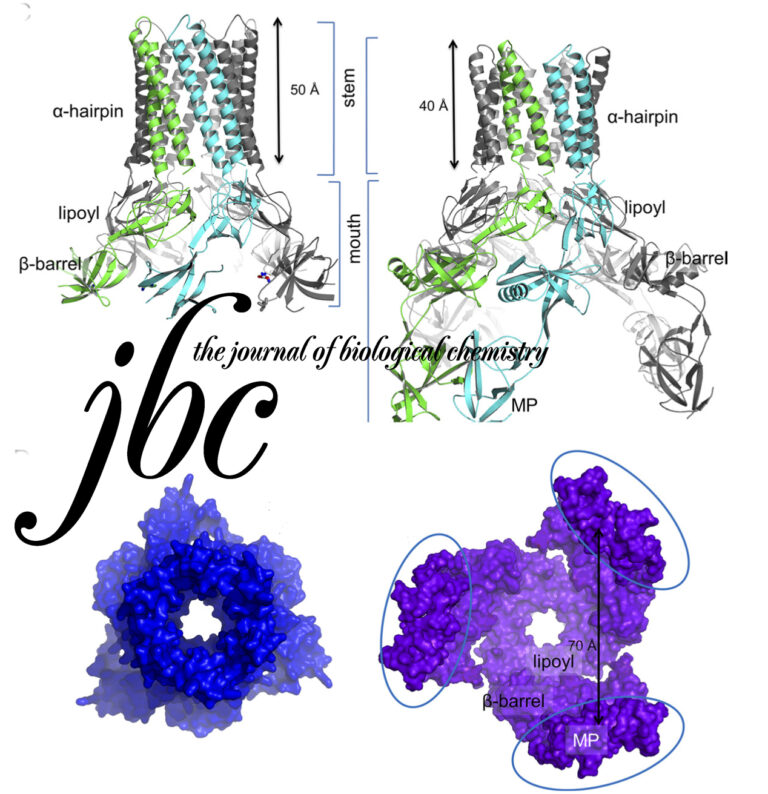
Funnel-like hexameric assembly of the periplasmic adapter protein in the tripartite multidrug efflux pump in gram-negative bacteria.
The Escherichia coli AcrAB-TolC pump is the principal multidrug exporter that confers intrinsic drug tolerance to the bacteria. The inner membrane transporter AcrB requires the outer membrane factor TolC and the periplasmic adapter protein AcrA. However, it remains ambiguous how the three proteins are assembled. In this study, a hexameric model of the adapter protein was generated based on the propensity for trimerization of a dimeric unit, and this model was further validated by presenting its channel-forming property that determines the substrate specificity.
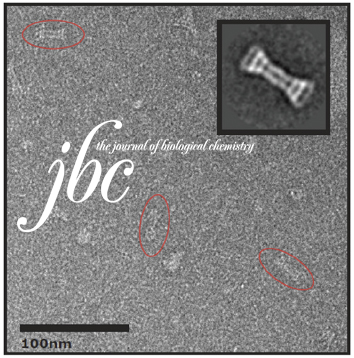
Initial evaluation of a direct detection device detector for single particle cryo-electron microscopy.
We report on initial results of using a new direct detection device (DDD) for single particle reconstruction of vitreous ice embedded specimens. Images were acquired on a Tecnai F20 at 200 keV and a nominal magnification of 29,000×.
Automation in Single-Particle Electron Microscopy Cryo-EM, Part C: Analyses, Interpretation, and Case studies
Throughout the history of single-particle electron microscopy (EM), automated technologies have seen varying degrees of emphasis and development, usually depending upon the contemporary demands of the field. We are currently faced with increasingly sophisticated devices for specimen preparation, vast increases in the size of collected data sets, comprehensive algorithms for image processing, sophisticated tools for quality assessment, and an influx of interested scientists from outside the field who might lack the skills of experienced microscopists.

10-A cryoEM structure and molecular model of the Myriapod (Scutigera) 6x6mer hemocyanin: understanding a giant oxygen transport protein.
Oxygen transport in Myriapoda is maintained by a unique 6 × 6mer hemocyanin, that is, 36 subunits arranged as six hexamers (1 × 6mers). In the sluggish diplopod Spirostreptus, the 1 × 6mers seem to operate as almost or fully independent allosteric units (h ∼ 1.3; P50 ∼ 5 torr), whereas in the swift centipede Scutigera, they intensively cooperate allosterically (h ∼ 10; P50 ∼ 50 torr).
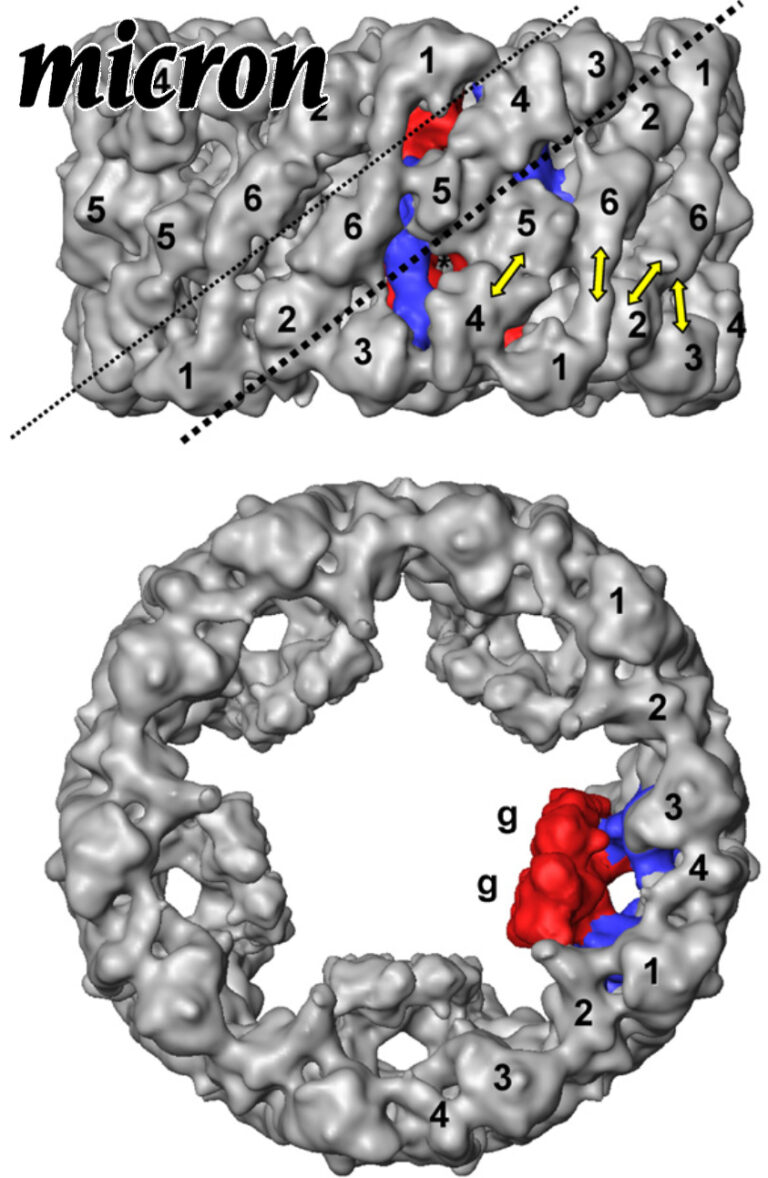
Comparative 11A structure of two molluscan hemocyanins from 3D cryo-electron microscopy.
Hemocyanins are giant extracellular proteins that transport oxygen in the hemolymph of many molluscs. Molluscan hemocyanins are cylindrical decamers or didecamers of a 350–400 kDa subunit that contains seven or eight different covalently linked globular functional units (FUs), arranged in a linear manner. Each FU carries a single copper active site and reversibly binds one dioxygen molecule. As a consequence, the decamer can carry up to 70 or 80 O2 molecules.

Nautilus pompilius hemocyanin: 9 A cryo-EM structure and molecular model reveal the subunit pathway and the interfaces between the 70 functional units.
Hemocyanins are giant extracellular oxygen carriers in the hemolymph of many molluscs. Nautilus pompilius (Cephalopoda) hemocyanin is a cylindrical decamer of a 350 kDa polypeptide subunit that in turn is a “pearl-chain” of seven different functional units (FU-a to FU-g). Each globular FU has a binuclear copper centre that reversibly binds one O2 molecule, and the 70-FU decamer is a highly allosteric protein. Its primary structure and an 11 Å cryo-electron microscopy (cryo-EM) structure have recently been determined, and the crystal structures of two related FU types are available in the databanks.