Vanessa Moeller, Ralf Dürr, Ladan Sarraf-Zadeh, Sabrina Keller, Stefanie Heinz, Nadja Hellmann, Arne Moeller, Bernhard Lieb, Jürgen Markl
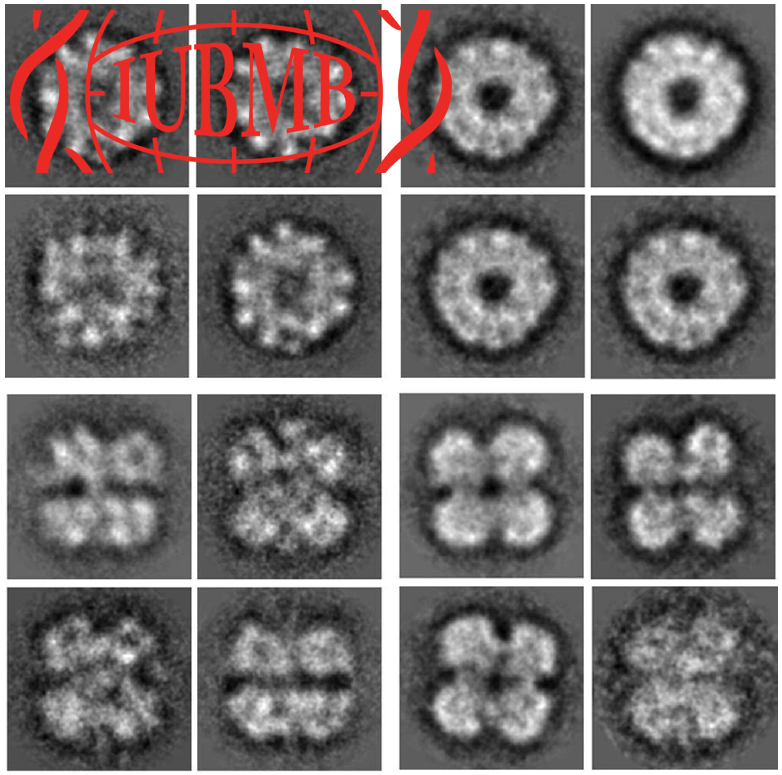
The extracellular hemoglobin multimer of the planorbid snail Biomphalaria glabrata , intermediate host of the human parasite Schistosoma mansoni , is presumed to be a 1.44 MDa complex of six 240 kDa polypeptide subunits, arranged as three disulfide‐bridged dimers. The complete amino acid sequence of two subunit types (BgHb1 and BgHb2), and the partial sequence of a third type (BgHb3) are known. Each subunit encompasses 13 paralogus heme domains, and N‐terminally a smaller plug domain responsible for subunit dimerization. We report here the recombinant expression of different functional fragments of BgHb2 in Escherichia coli , and of the complete functional subunits BgHb1 and BgHb2 in insect cells; BgHb1 was also expressed as disulfide‐bridged dimer (480 kDa). Oxygen‐binding measurements of the recombinant products show a P50 of about 7 mmHg and the absence of a significant cooperativity or Bohr effect. The covalently linked dimer of BgHb1, but not the monomer, is capable to form aggregates closely resembling native BgHb molecules in the electron microscope.
DOI: 10.1002/iub.453
PMID: 21491558