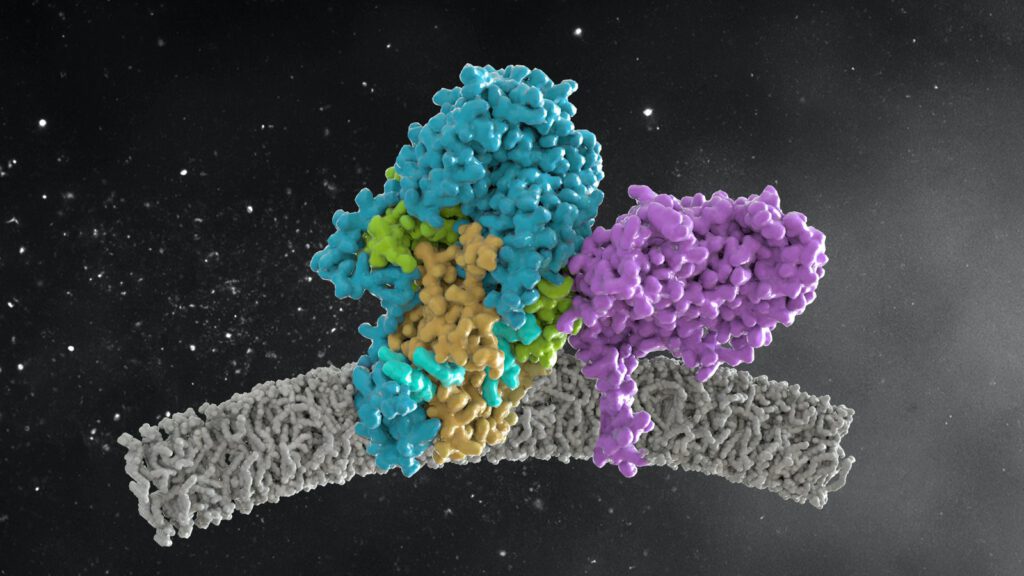
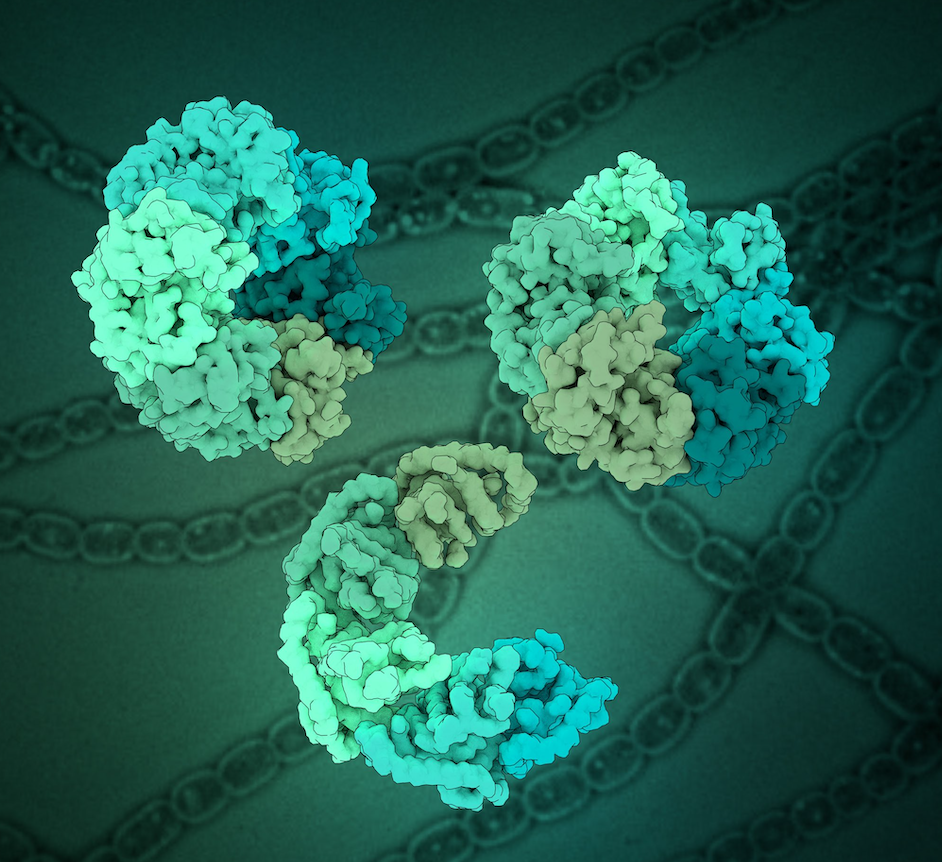
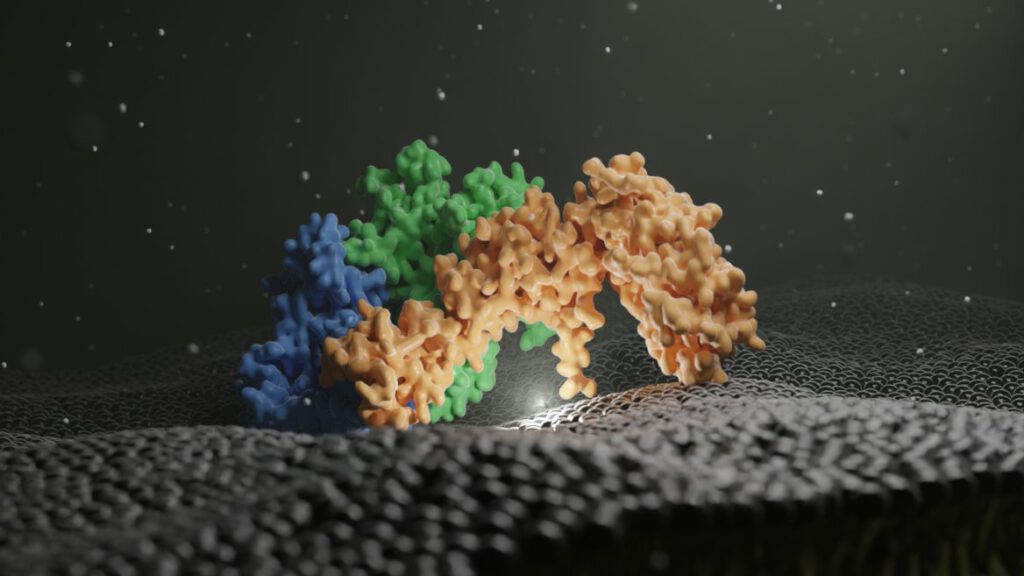
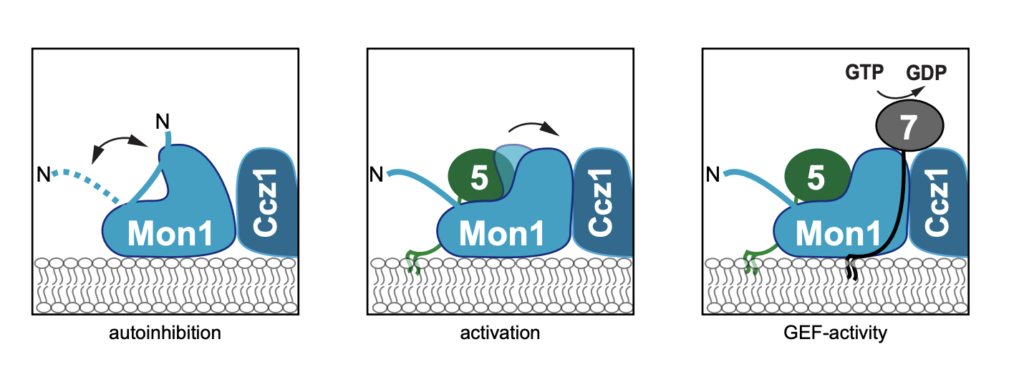
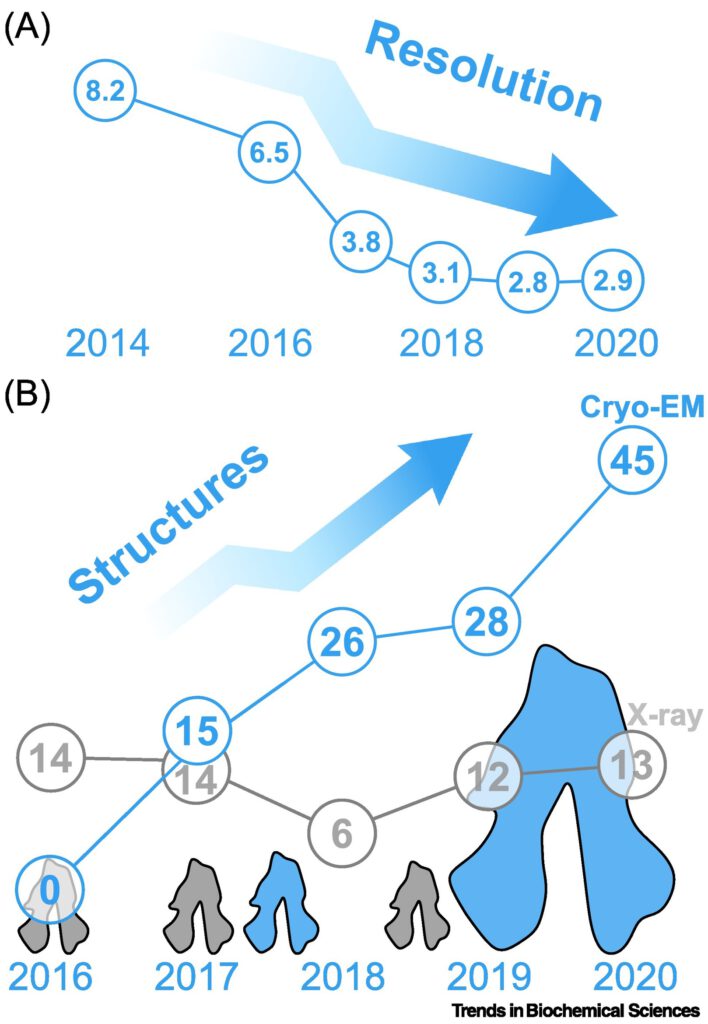
Structure of the ceramide-bound SPOTS complex
Jan-Hannes Schäfer, Carolin Körner, Bianca M. Esch, Sergej Limar, KristianParey, Stefan Walter, Dovile Januliene#, Arne Moeller#, Florian Fröhlich# Sphingolipids are structural membrane components that also function in cellular stress responses. The serine palmitoyl-transferase (SPT) catalyzes the rate limiting step in sphingolipid biogenesis. Its activity is tightly regulated through multiple binding partners, including Tsc3, Orm proteins, […]
Structure of the ceramide-bound SPOTS complex Read More »