Linlin Yang, Dehua Yang, Chris de Graaf, Arne Moeller, Graham M. West, Venkatasubramanian Dharmarajan, Chong Wang, Fai Y. Siu, Gaojie Song, Steffen Reedtz-Runge, Bruce D. Pascal, Beili Wu, Clinton S. Potter, Hu Zhou, Patrick R. Griffin, Bridget Carragher, Huaiyu Yang, Ming-Wei Wang, Raymond C. Stevens & Hualiang Jiang
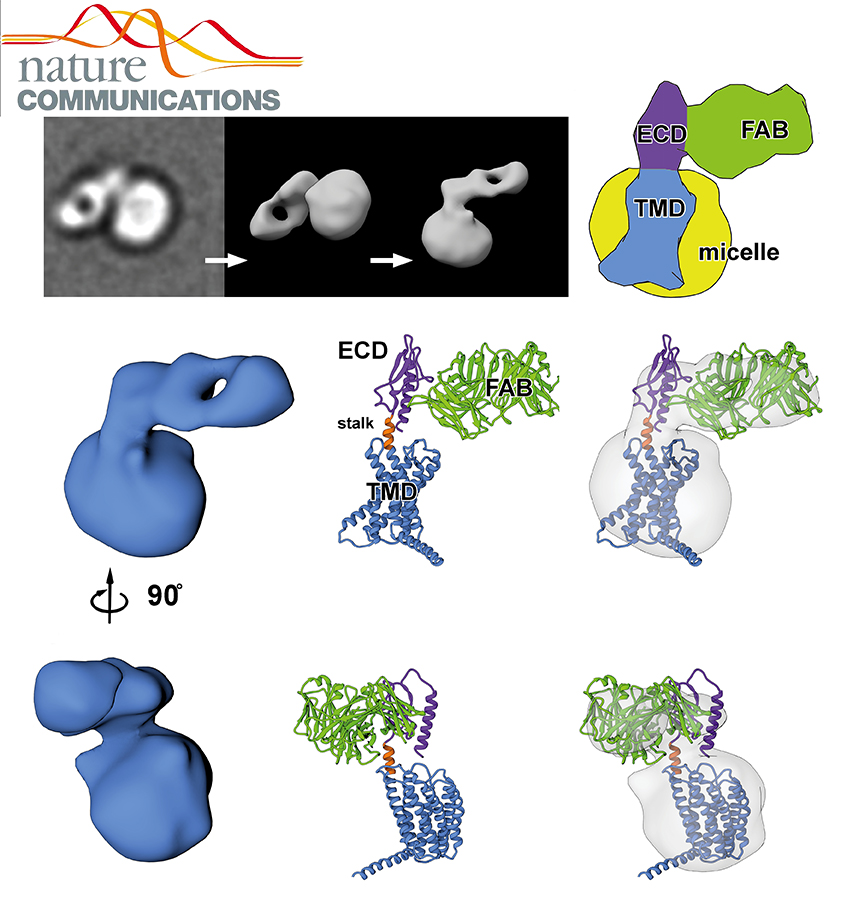
Class B G protein-coupled receptors are composed of an extracellular domain (ECD) and a seven-transmembrane (7TM) domain, and their signalling is regulated by peptide hormones. Using a hybrid structural biology approach together with the ECD and 7TM domain crystal structures of the glucagon receptor (GCGR), we examine the relationship between full-length receptor conformation and peptide ligand binding. Molecular dynamics (MD) and disulfide crosslinking studies suggest that apo-GCGR can adopt both an open and closed conformation associated with extensive contacts between the ECD and 7TM domain. The electron microscopy (EM) map of the full-length GCGR shows how a monoclonal antibody stabilizes the ECD and 7TM domain in an elongated conformation. Hydrogen/deuterium exchange (HDX) studies and MD simulations indicate that an open conformation is also stabilized by peptide ligand binding. The combined studies reveal the open/closed states of GCGR and suggest that glucagon binds to GCGR by a conformational selection mechanism.
DOI: 10.1038/ncomms8859
PMID: 26227798