Division of labor in transhydrogenase by alternating proton translocation and hydride transfer.
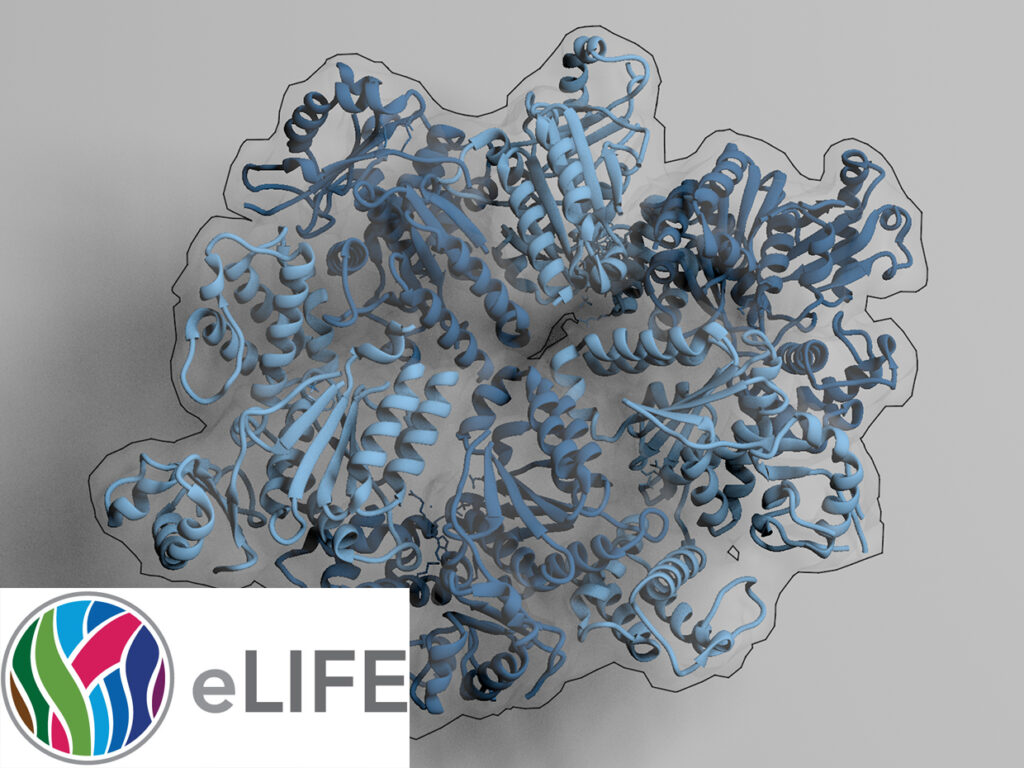
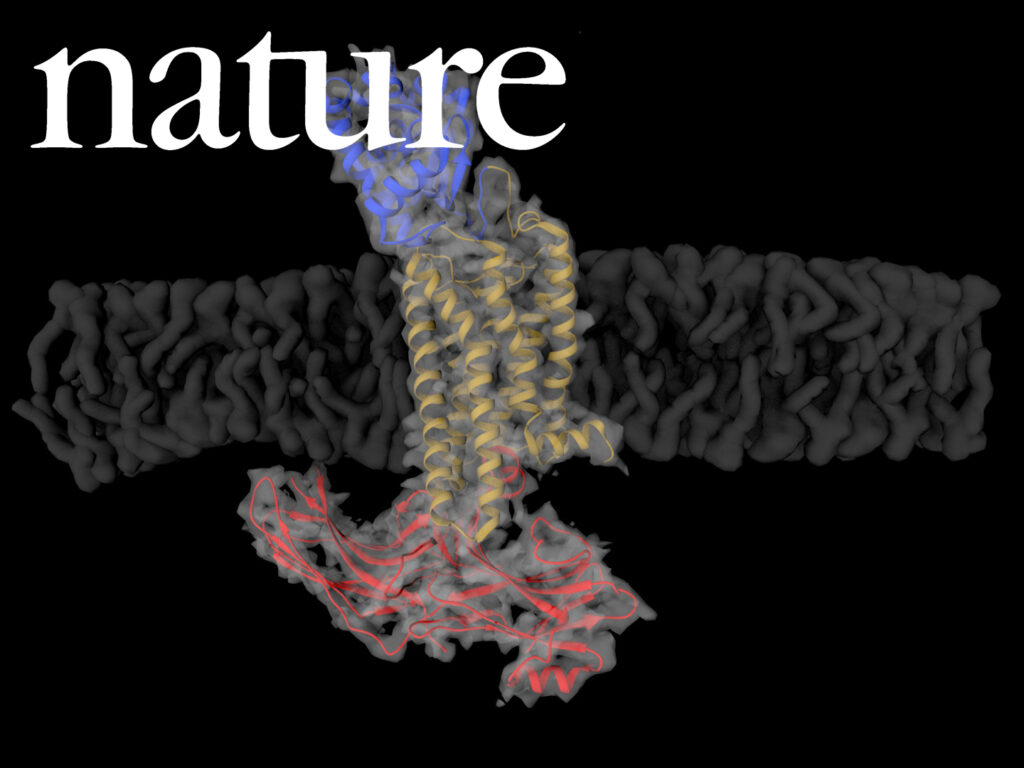
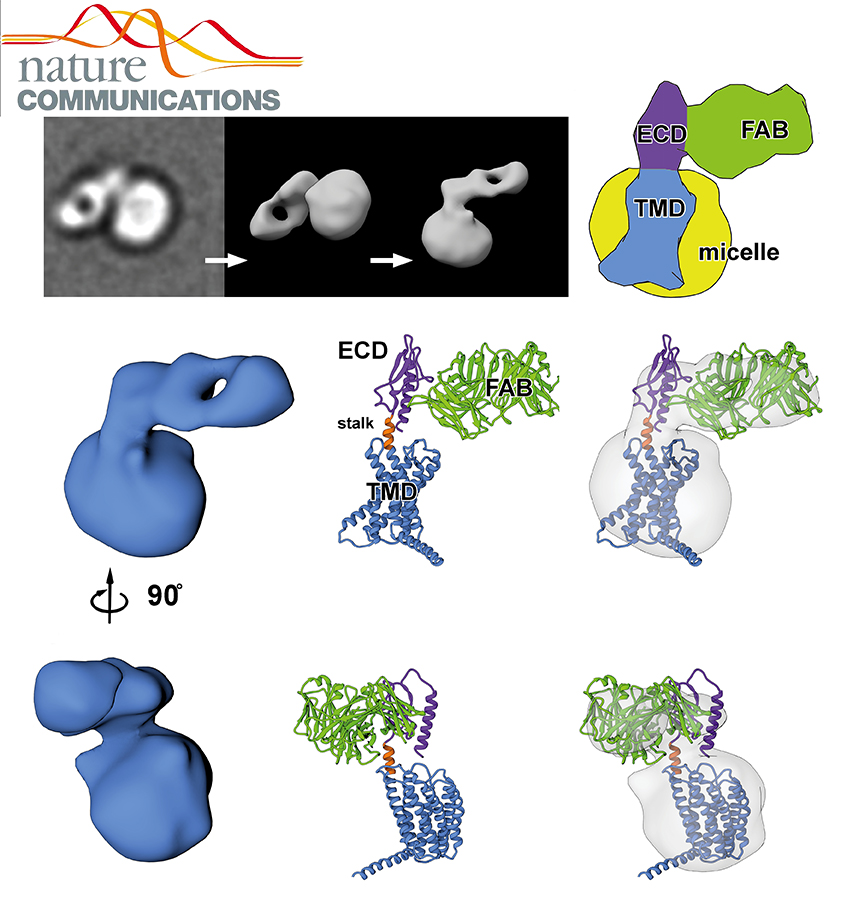
NADPH/NADP+ (the reduced form of NADP+/nicotinamide adenine dinucleotide phosphate) homeostasis is critical for countering oxidative stress in cells. Nicotinamide nucleotide transhydrogenase (TH), a membrane enzyme present in both bacteria and mitochondria, couples the proton motive force to the generation of NADPH. We present the 2.8 Å crystal structure of the transmembrane proton channel domain of TH from Thermus thermophilus and the 6.9 Å crystal structure of the entire enzyme (holo-TH).