Theresa Gewering, Deepali Waghray, Kristian Parey, Joel Zapata, Pengyi Zhao, Hao Chen, Dovile Januliene, Ina L. Urbatsch, Arne Moeller, Qinghai Zhang
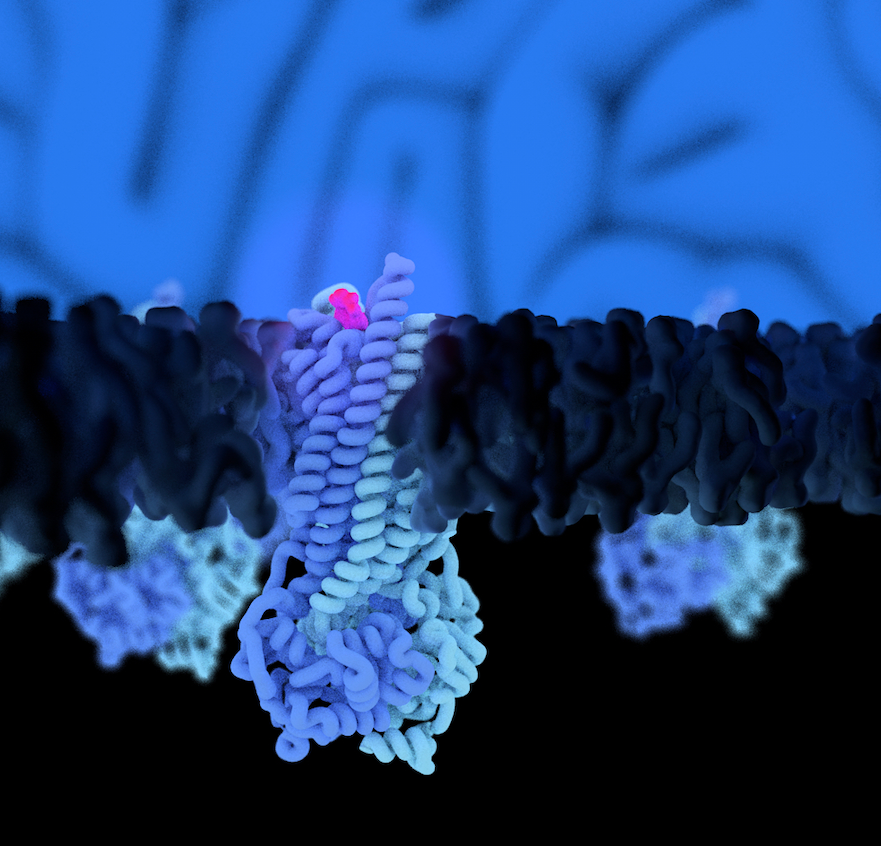
P-glycoprotein (Pgp) is a highly dynamic ATP-binding cassette transporter that confers cancer multidrug resistance and mediates the bioavailability and pharmacokinetics of many drugs. Structural and biochemical data have provided insights into the binding of diverse compounds, but how they are translocated through the membrane has remained elusive. The biggest hurdle to address this fundamental question has been isolating and characterizing the short-lived transition states during transport. Here, we covalently attached a cyclopeptide substrate to discrete sites of Pgp. Cryo-EM analyses of inward- and outward-facing conformations enabled tracing of the substrate passage and revealed how a cascade of conformational changes in transmembrane helix 1 drives substrate movement across the membrane bilayer upon ATP binding. Observation of a double substrate-bound outward-facing conformation suggests that Pgp can co-transport two compounds simultaneously.