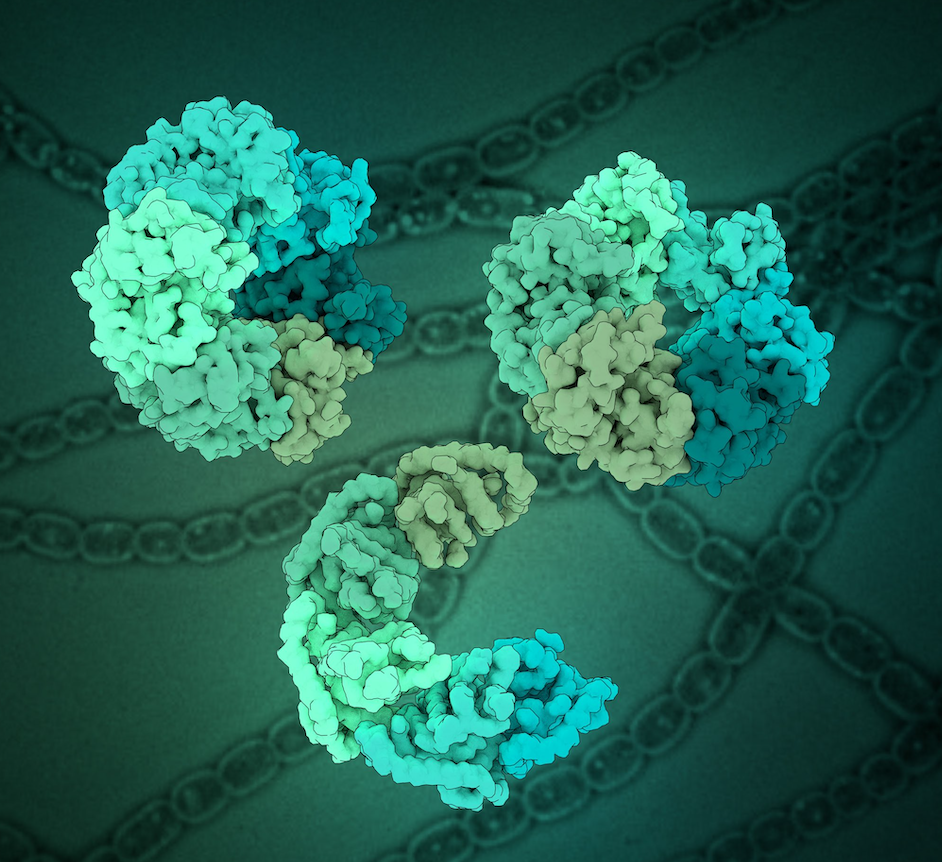
Dmitry Shvarev, Alischa Ira Scholz, Arne Moeller
Magnesium chelatase is a conserved enzyme complex responsible for the first committed step of chlorophyll biosynthesis in photosynthetic organisms, which is the addition of magnesium to the chlorophyll precursor, protoporphyrin IX. The complex is composed of the catalytic subunit ChlH, the bridging subunit ChlD, and the subunit ChlI, which serves as the motor that drives the entire complex. Although the enzyme is well-characterized functionally, high-resolution structures are available only for individual subunits. Hence, the full assembly and the molecular mechanism of the enzyme complex remains unknown. Here, we used cryo-EM, supported by biochemical analysis and mass photometry, to determine structures of the ChlI motor subunit of magnesium chelatase under turnover conditions in the presence of ATP. Our data reveal the molecular details of ChlI oligomerization and conformational dynamics upon ATP binding and hydrolysis. These findings provide new insights into the mechanistic function of ChlI and its implications for the entire magnesium chelatase complex machinery.