Ann-Christin Borchers, Maren Janz, Jan-Hannes Schäfer, Arne Moeller, Daniel Kümmel, Achim Paululat, Christian Ungermann, Lars Langemeyer
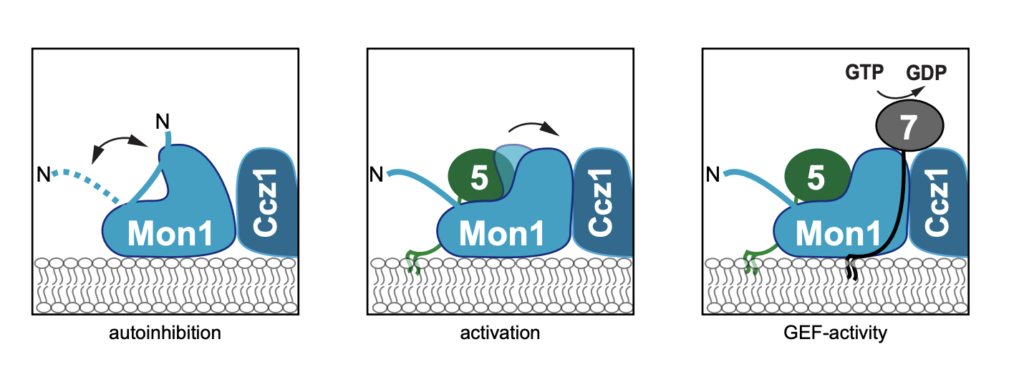
Maturation from early to late endosomes depends on the exchange of their marker proteins Rab5 to Rab7. This requires Rab7 activation by its specific guanine nucleotide exchange factor (GEF) Mon1- Ccz1. Efficient GEF activity of this complex on membranes depends on Rab5, thus driving Rabexchange on endosomes. However, molecular details on the role of Rab5 in Mon1-Ccz1 activation are unclear. Here we identify key features in Mon1 involved in GEF regulation. We show that the intrinsically disordered N-terminal domain of Mon1 autoinhibits Rab5-dependent GEF-activity on membranes. Consequently, Mon1 truncations result in higher GEF activity in vitro, and a shift from Rab5 to more Rab7 positive structures in Drosophila nephrocytes and yeast, suggesting faster endosomal maturation. Using modeling, we further identify a conserved Rab5 binding site in Mon1. Mutations impairing Rab5 interaction result in poor GEF activity on membranes and growth defects in vivo. Our analysis provides a framework to understand the mechanism of Rab-conversion and organelle maturation along the endomembrane system.
BiorXiv: doi.org/10.1101/2023.03.01.530579