Structure of the endosomal CORVET tethering complex
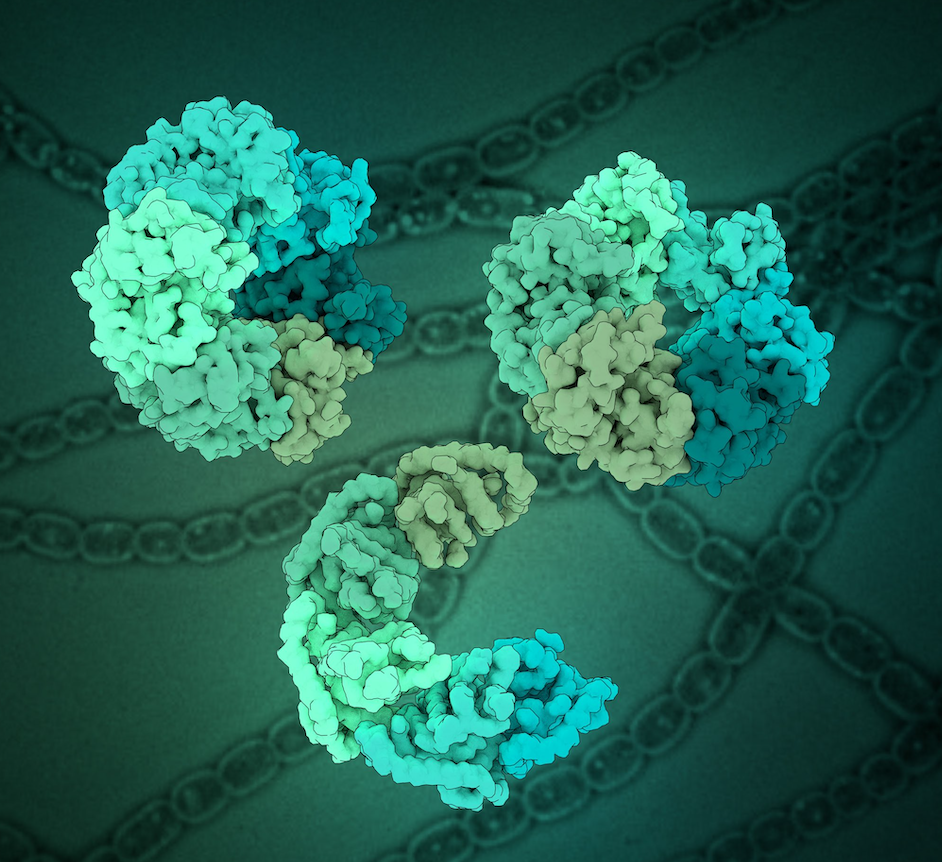
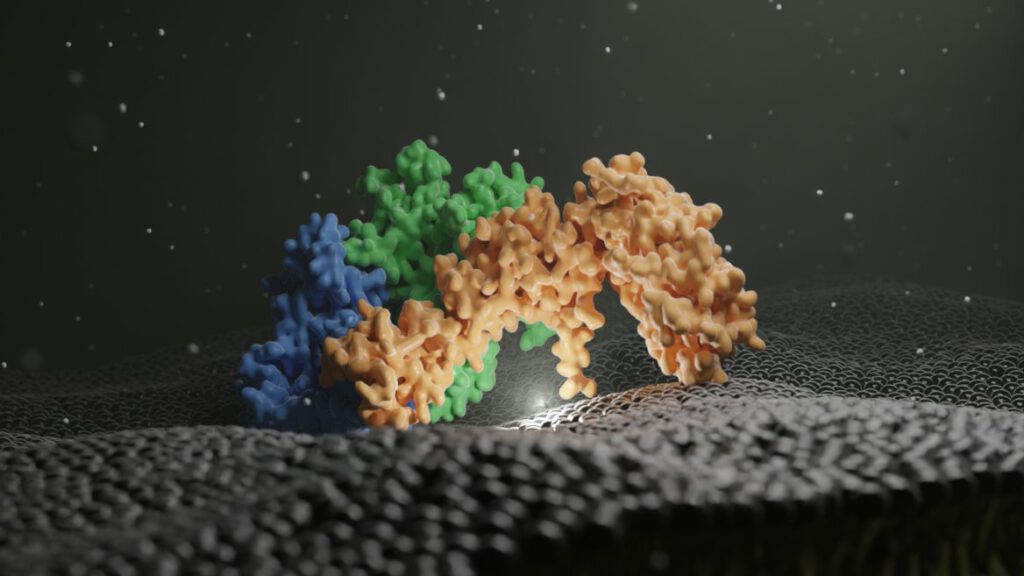
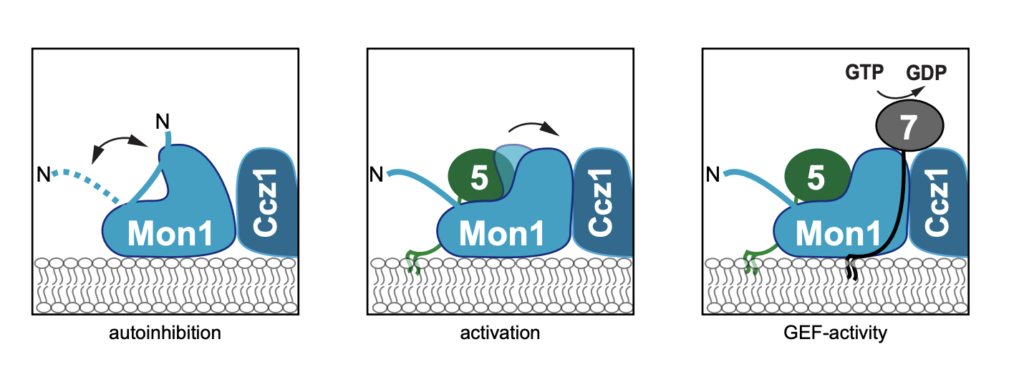
Dmitry Shvarev, Caroline König, Nicole Susan, Lars Langemeyer, Stefan Walter, Angela Perz, Florian Fröhlich, Christian Ungermann, Arne Moeller Cells depend on their endolysosomal system for nutrient uptake and downregulation of plasma membrane proteins. These processes rely on endosomal maturation, which requires multiple membrane fusion steps. Early endosome fusion is promoted by the Rab5 GTPase and […]
Structure of the endosomal CORVET tethering complex Read More »