TRIP13 is a protein-remodeling AAA+ ATPase that catalyzes MAD2 conformation switching.
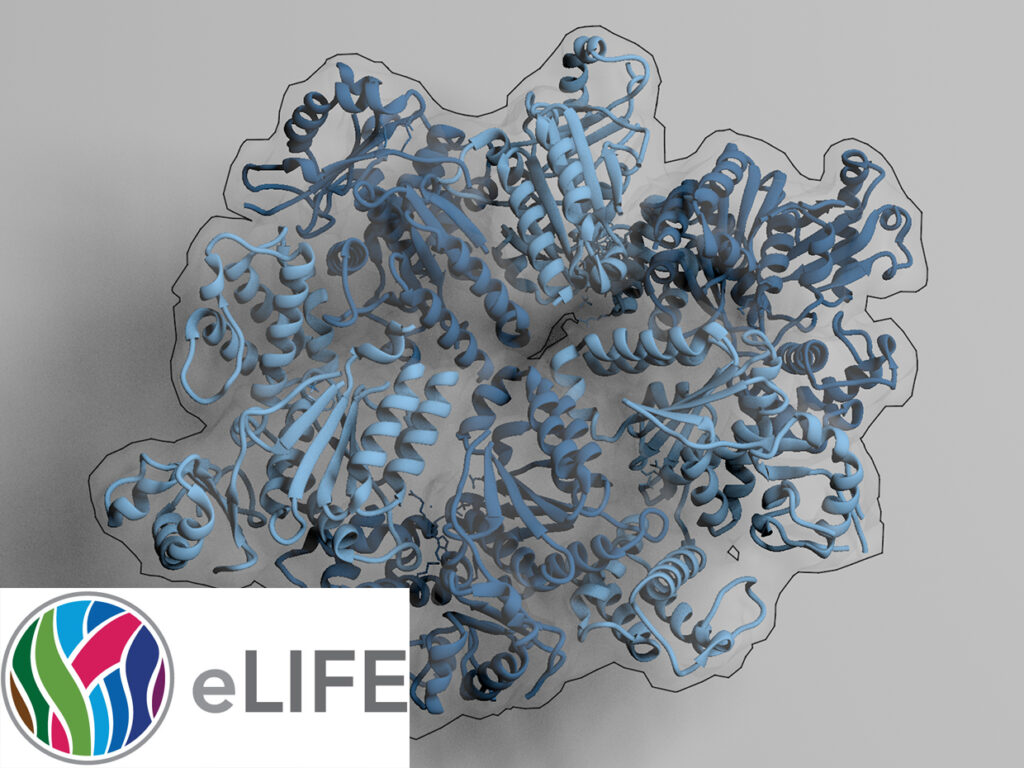
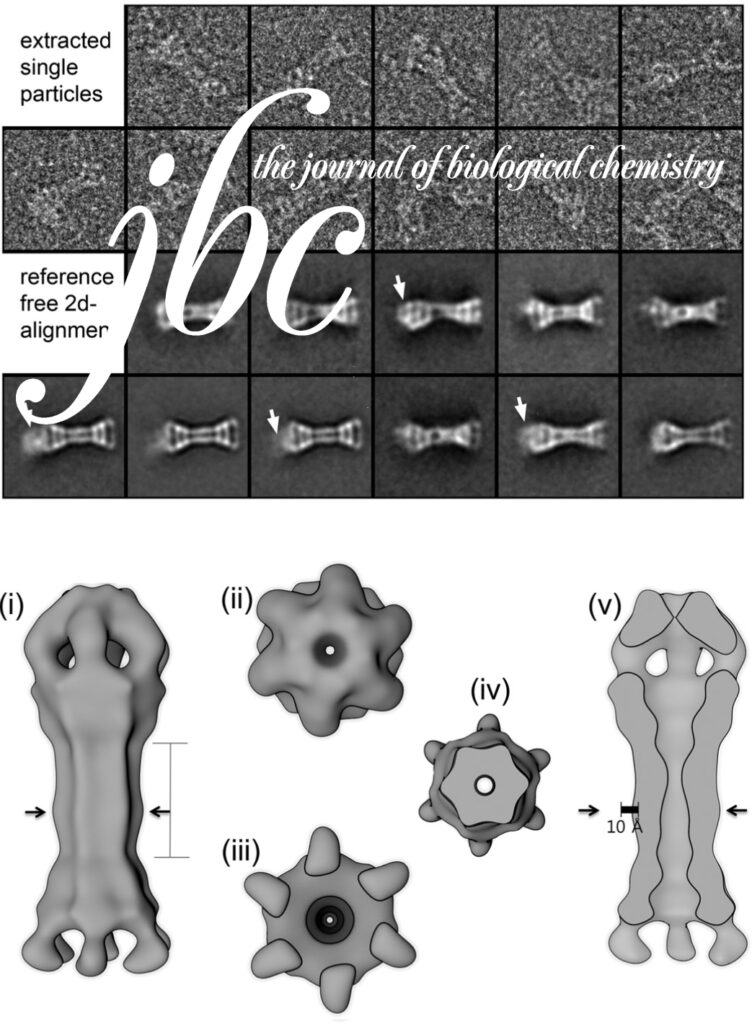
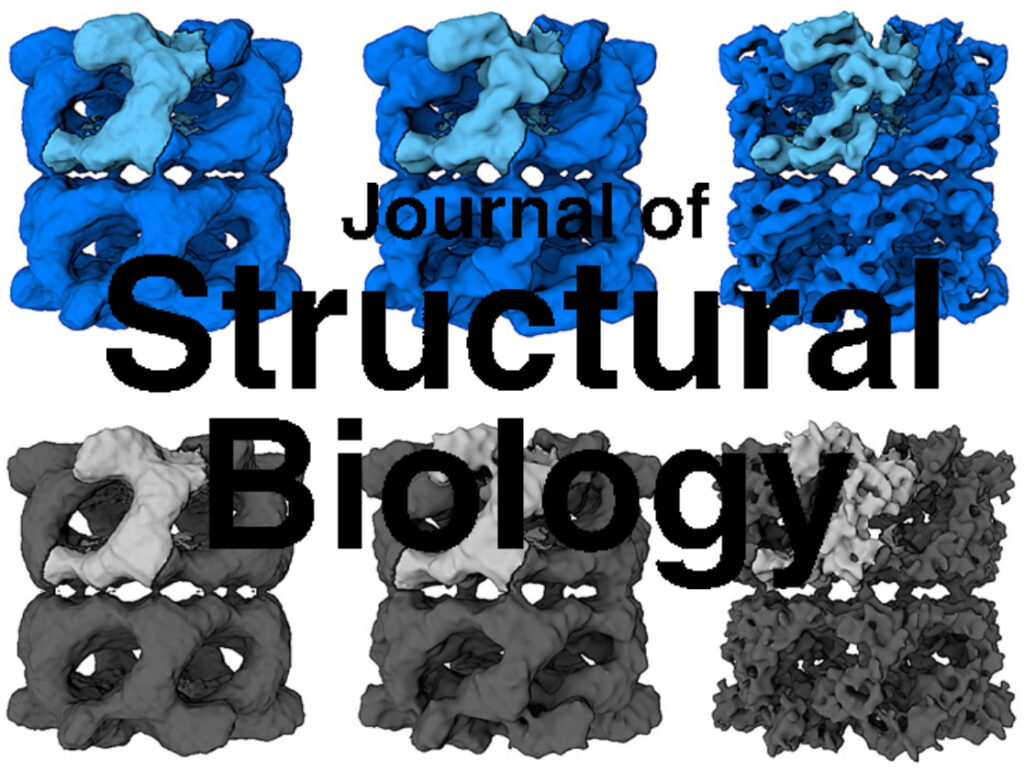
The AAA+ family ATPase TRIP13 is a key regulator of meiotic recombination and the spindle assembly checkpoint, acting on signaling proteins of the conserved HORMA domain family. Here we present the structure of the Caenorhabditis elegans TRIP13 ortholog PCH-2, revealing a new family of AAA+ ATPase protein remodelers.
TRIP13 is a protein-remodeling AAA+ ATPase that catalyzes MAD2 conformation switching. Read More »