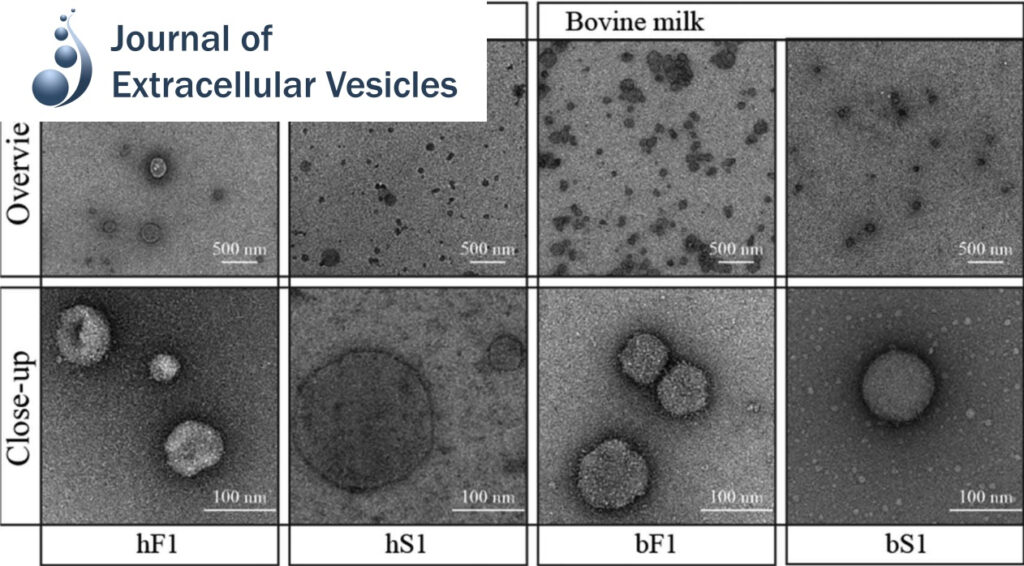
Acidic Environment Induces Dimerization and Ligand Binding Site Collapse in the Vps10p Domain of Article Acidic Environment Induces Dimerization and Ligand Binding Site Collapse in the Vps10p Domain of Sortilin
Sortilin is a neuronal receptor involved in transmembrane signaling, endocytosis, and intracellular sorting of proteins. It cycles through a number of cellular compartments where it encounters various acidic conditions. The crystal structure of the sortilin ectodomain has previously been determined at neutral pH. Here, we present the 3.5-Å resolution crystal structure of sortilin at pH 5.5, which represents an environment similar to that of late endosomes, where ligands are released.